Erlotinib and bevacizumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck: a phase I/II study
- PMID: 19201650
- PMCID: PMC2768532
- DOI: 10.1016/S1470-2045(09)70002-6
Erlotinib and bevacizumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck: a phase I/II study
Abstract
Background: Epidermal growth factor receptor (EGFR) is a validated target in squamous-cell carcinoma of the head and neck, but in patients with recurrent or metastatic disease, EGFR targeting agents have displayed modest efficacy. Vascular endothelial growth factor (VEGF)-mediated angiogenesis has been implicated as a mechanism of resistance to anti-EGFR therapy. In this multi-institutional phase I/II study we combined an EGFR inhibitor, erlotinib, with an anti-VEGF antibody, bevacizumab.
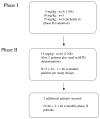
Methods: Between April 15, 2003, and Jan 27, 2005, patients with recurrent or metastatic squamous-cell carcinoma of the head and neck were enrolled from seven centres in the USA and were given erlotinib (150 mg daily) and bevacizumab in escalating dose cohorts. The primary objectives in the phase I and II sections, respectively, were to establish the maximum tolerated dose and dose-limiting toxicity of bevacizumab when administered with erlotinib and to establish the proportion of objective responses and time to disease progression. Pretreatment serum and tissues were collected and analysed by enzyme-linked immunosorbent assay and immunofluorescence quantitative laser analysis, respectively. This study was registered with ClinicalTrials.gov, number NCT00055913.
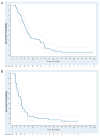
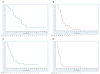
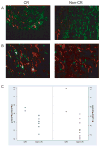
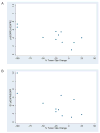
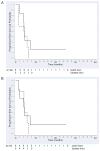
Findings: In the phase I section of the trial, ten patients were enrolled in three successive cohorts with no dose-limiting toxic effects noted. 46 patients were enrolled in the phase II section of the trial (including three patients from the phase I section) on the highest dose of bevacizumab (15 mg/kg every 3 weeks). Two additional patients were accrued beyond the protocol-stipulated 46, leaving a total of 48 patients for the phase II assessment. The most common toxic effects of any grade were rash and diarrhoea (41 and 16 of 48 patients, respectively). Three patients had serious bleeding events of grade 3 or higher. Seven patients had a response, with four showing a complete response allowing rejection of the null hypothesis. Median time of overall survival and progression-free survival (PFS) were 7.1 months (95% CI 5.7-9.0) and 4.1 months (2.8-4.4), respectively. Higher ratios of tumour-cell phosphorylated VEGF receptor-2 (pVEGFR2) over total VEGFR2 and endothelial-cell pEGFR over total EGFR in pretreatment biopsies were associated with complete response (0.704 vs 0.386, p=0.036 and 0.949 vs 0.332, p=0.036, respectively) and tumour shrinkage (p=0.007 and p=0.008, respectively) in a subset of 11 patients with available tissue.
Interpretation: The combination of erlotinib and bevacizumab is well tolerated in recurrent or metastatic squamous-cell carcinoma of the head and neck. A few patients seem to derive a sustained benefit and complete responses were associated with expression of putative targets in pretreatment tumour tissue.
Conflict of interest statement
The authors declared no conflicts of interest.
Figures
Comment in
-
Targeting the future in head and neck cancer.Lancet Oncol. 2009 Mar;10(3):204-5. doi: 10.1016/S1470-2045(09)70051-8. Lancet Oncol. 2009. PMID: 19261251 No abstract available.
References
-
- Pisani P, Bray F, Parkin DM. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int J Cancer. 2002;97:72–81. - PubMed
-
- Ahmed SM, Cohen EE. Treatment of squamous cell carcinoma of the head and neck in the metastatic and refractory settings: advances in chemotherapy and the emergence of small molecule epidermal growth factor receptor kinase inhibitors. Current cancer drug targets. 2007;7:666–73. - PubMed
-
- Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–27. - PubMed
-
- Cohen EE. Role of epidermal growth factor receptor pathway-targeted therapy in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2006;24:2659–65. - PubMed
-
- Ciardiello F, Bianco R, Damiano V, Fontanini G, Caputo R, Pomatico G, et al. Antiangiogenic and antitumor activity of anti-epidermal growth factor receptor C225 monoclonal antibody in combination with vascular endothelial growth factor antisense oligonucleotide in human GEO colon cancer cells. Clin Cancer Res. 2000;6:3739–47. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

