Adalimumab for the treatment of fistulas in patients with Crohn's disease
- PMID: 19201775
- PMCID: PMC2689393
- DOI: 10.1136/gut.2008.159251
Adalimumab for the treatment of fistulas in patients with Crohn's disease
Abstract
Objective: To evaluate the efficacy of adalimumab in the healing of draining fistulas in patients with active Crohn's disease (CD).
Design: A phase III, multicentre, randomised, double-blind, placebo controlled study with an open-label extension was conducted in 92 sites.
Patients: A subgroup of adults with moderate to severely active CD (CD activity index 220-450) for >or=4 months who had draining fistulas at baseline.
Interventions: All patients received initial open-label adalimumab induction therapy (80 mg/40 mg at weeks 0/2). At week 4, all patients were randomly assigned to receive double-blind placebo or adalimumab 40 mg every other week or weekly to week 56 (irrespective of fistula status). Patients completing week 56 of therapy were then eligible to enroll in an open-label extension.
Main outcome measures: Complete fistula healing/closure (assessed at every visit) was defined as no drainage, either spontaneous or with gentle compression.
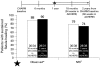
Results: Of 854 patients enrolled, 117 had draining fistulas at both screening and baseline (70 randomly assigned to adalimumab and 47 to placebo). The mean number of draining fistulas per day was significantly decreased in adalimumab-treated patients compared with placebo-treated patients during the double-blind treatment period. Of all patients with healed fistulas at week 56 (both adalimumab and placebo groups), 90% (28/31) maintained healing following 1 year of open-label adalimumab therapy (observed analysis).
Conclusions: In patients with active CD, adalimumab therapy was more effective than placebo for inducing fistula healing. Complete fistula healing was sustained for up to 2 years by most patients in an open-label extension trial.
Trial registration: ClinicalTrials.gov NCT00077779 NCT00195715.
Figures







Comment in
-
Adalimumab for the treatment of fistulas in patients with Crohn's disease.Inflamm Bowel Dis. 2011 Feb;17(2):667-8. doi: 10.1002/ibd.21343. Inflamm Bowel Dis. 2011. PMID: 20848525 No abstract available.
References
-
- Schwartz DA, Pemberton JH, Sandborn WJ. Diagnosis and treatment of perianal fistulas in Crohn disease. Ann Intern Med 2001;135:906–18 - PubMed
-
- Schwartz DA, Loftus EV, Jr, Tremaine WJ, et al. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology 2002;122:875–80 - PubMed
-
- Lapidus A, Bernell O, Hellers G, et al. Clinical course of colorectal Crohn’s disease: a 35-year follow-up study of 507 patients. Gastroenterology 1998;114:1151–60 - PubMed
-
- Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med 1999;340:1398–405 - PubMed
-
- Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med 2004;350:876–85 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
