Crystal structure of butyrate kinase 2 from Thermotoga maritima, a member of the ASKHA superfamily of phosphotransferases
- PMID: 19201797
- PMCID: PMC2668392
- DOI: 10.1128/JB.00906-08
Crystal structure of butyrate kinase 2 from Thermotoga maritima, a member of the ASKHA superfamily of phosphotransferases
Retraction in
-
Retraction. Crystal structure of butyrate kinase 2 from Thermotoga maritima, a member of the ASKHA superfamily of phosphotransferases.J Bacteriol. 2012 Jun;194(11):3033. doi: 10.1128/JB.00549-12. J Bacteriol. 2012. PMID: 22582386 Free PMC article. No abstract available.
Abstract
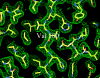
The enzymatic transfer of phosphoryl groups is central to the control of many cellular processes. One of the phosphoryl transfer mechanisms, that of acetate kinase, is not completely understood. Besides better understanding of the mechanism of acetate kinase, knowledge of the structure of butyrate kinase 2 (Buk2) will aid in the interpretation of active-site structure and provide information on the structural basis of substrate specificity. The gene buk2 from Thermotoga maritima encodes a member of the ASKHA (acetate and sugar kinases/heat shock cognate/actin) superfamily of phosphotransferases. The encoded protein Buk2 catalyzes the phosphorylation of butyrate and isobutyrate. We have determined the 2.5-A crystal structure of Buk2 complexed with (beta,gamma-methylene) adenosine 5'-triphosphate. Buk2 folds like an open-shelled clam, with each of the two domains representing one of the two shells. In the open active-site cleft between the N- and C-terminal domains, the active-site residues consist of two histidines, two arginines, and a cluster of hydrophobic residues. The ATP binding region of Buk2 in the C-terminal domain consists of abundant glycines for nucleotide binding, and the ATP binding motif is similar to those of other members of the ASKHA superfamily. The enzyme exists as an octamer, in which four disulfide bonds form between intermolecular cysteines. Sequence alignment and structure superposition identify the simplicity of the monomeric Buk2 structure, a probable substrate binding site, the key residues in catalyzing phosphoryl transfer, and the substrate specificity differences among Buk2, acetate, and propionate kinases. The possible enzyme mechanisms are discussed.
Figures
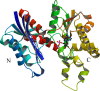

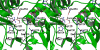
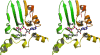



Similar articles
-
Crystallization of butyrate kinase 2 from Thermotoga maritima mediated by vapor diffusion of acetic acid.Acta Crystallogr D Biol Crystallogr. 2003 Jun;59(Pt 6):1100-2. doi: 10.1107/s0907444903007832. Epub 2003 May 23. Acta Crystallogr D Biol Crystallogr. 2003. PMID: 12777787
-
Crystal structures of ADP and AMPPNP-bound propionate kinase (TdcD) from Salmonella typhimurium: comparison with members of acetate and sugar kinase/heat shock cognate 70/actin superfamily.J Mol Biol. 2005 Sep 30;352(4):876-92. doi: 10.1016/j.jmb.2005.07.069. J Mol Biol. 2005. PMID: 16139298
-
Structural bases of feed-back control of arginine biosynthesis, revealed by the structures of two hexameric N-acetylglutamate kinases, from Thermotoga maritima and Pseudomonas aeruginosa.J Mol Biol. 2006 Feb 24;356(3):695-713. doi: 10.1016/j.jmb.2005.11.079. Epub 2005 Dec 12. J Mol Biol. 2006. PMID: 16376937
-
Structures of substrate- and nucleotide-bound propionate kinase from Salmonella typhimurium: substrate specificity and phosphate-transfer mechanism.Acta Crystallogr D Biol Crystallogr. 2015 Aug;71(Pt 8):1640-8. doi: 10.1107/S1399004715009992. Epub 2015 Jul 28. Acta Crystallogr D Biol Crystallogr. 2015. PMID: 26249345
-
Mechanistic features of Salmonella typhimurium propionate kinase (TdcD): insights from kinetic and crystallographic studies.Biochim Biophys Acta. 2013 Oct;1834(10):2036-44. doi: 10.1016/j.bbapap.2013.05.020. Epub 2013 Jun 5. Biochim Biophys Acta. 2013. PMID: 23747922
Cited by
-
Employing a recombinant strain of Advenella mimigardefordensis for biotechnical production of Homopolythioesters from 3,3'-dithiodipropionic acid.Appl Environ Microbiol. 2012 May;78(9):3286-97. doi: 10.1128/AEM.00007-12. Epub 2012 Feb 17. Appl Environ Microbiol. 2012. PMID: 22344658 Free PMC article.
-
Influence of fatty acid precursors, including food preservatives, on the growth and fatty acid composition of Listeria monocytogenes at 37 and 10degreesC.Appl Environ Microbiol. 2010 Mar;76(5):1423-32. doi: 10.1128/AEM.01592-09. Epub 2010 Jan 4. Appl Environ Microbiol. 2010. PMID: 20048057 Free PMC article.
-
Structure of 2-oxo-3-deoxygalactonate kinase from Klebsiella pneumoniae.Acta Crystallogr D Biol Crystallogr. 2011 Aug;67(Pt 8):678-89. doi: 10.1107/S0907444911021834. Epub 2011 Jul 12. Acta Crystallogr D Biol Crystallogr. 2011. PMID: 21795809 Free PMC article.
References
-
- Anthony, R. S., and L. B. Spector. 1972. Phosphorylated acetate kinase. Its isolation and reactivity. J. Biol. Chem. 2472120-2125. - PubMed
-
- Archer, S., S. F. Meng, J. Wu, J. Johnson, R. Tang, and R. Hodin. 1998. Butyrate inhibits colon carcinoma cell growth through two distinctive pathways. Surgery 124248-253. - PubMed
-
- Blattler, W. A., and J. R. Knowles. 1979. Stereochemical course of phosphokinases. The use of adenosine[γ-(S) 16O,17O,18O]triphosphate and the mechanistic consequences for the reactions catalyzed by glycerol kinase, hexokinase, pyruvate kinase, and acetate kinase. Biochemistry 183927-3933. - PubMed
-
- Brünger, A. T., P. D. Adams, G. M. Clore, W. L. Delano, P. Gros, R. W. Grosse-Kunstleve, J. S. Jiang, J. Kuszewski, M. Nilges, N. S. Pannu, R. J. Read, L. M. Rice, T. Simonson, and G. L. Warren. 1998. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54905-921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

