Requirement of B cells for generating CD4+ T cell memory
- PMID: 19201839
- PMCID: PMC2658628
- DOI: 10.4049/jimmunol.0802501
Requirement of B cells for generating CD4+ T cell memory
Abstract
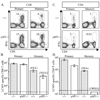
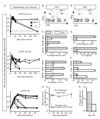
B cells can influence T cell responses by directly presenting Ag or by secreting Ab that binds to Ag to form immunogenic complexes. Conflicting evidence suggests that persisting Ag-Ab complexes propagate long-term T cell memory; yet, other data indicate that memory cells can survive without specific Ag or MHC. In this study, the roles of B cells and Ag-Ab complexes in T cell responses to lymphocytic choriomeningitis virus (LCMV) infection were investigated using B cell-deficient or B cell-competent mice. Despite normal lymphocyte expansion after acute infection, B cell-deficient mice rapidly lost CD4(+) T cell memory, but not CD8(+) T cell memory, during the contraction phase. To determine whether Ag-Ab complexes sustain CD4(+) T cell memory, T cell responses were followed in B cell-transgenic (mIg-Tg) mice that have B cells but neither LCMV-specific Ab nor LCMV-immune complex deposition. In contrast to B cell-deficient mice, mIg-Tg mice retained functional Th cell memory, indicating that B cells selectively preserve CD4(+) T cell memory independently of immune complex formation. An in vivo consequence of losing CD4(+) T cell memory was that B cell-deficient mice were unable to resolve chronic virus infection. These data implicate a B cell function other than Ab production that induces long-term protective immunity.
Figures




References
-
- Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–842. - PubMed
-
- Rogers PR, Dubey C, Swain SL. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J Immunol. 2000;164:2338–2346. - PubMed
-
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. - PubMed
Publication types
MeSH terms
Grants and funding
- T32 AI007019/AI/NIAID NIH HHS/United States
- R-01 CA038350/CA/NCI NIH HHS/United States
- R-01 AI43603/AI/NIAID NIH HHS/United States
- R01 CA038350/CA/NCI NIH HHS/United States
- R-01 AI074862/AI/NIAID NIH HHS/United States
- R37 AI030048/AI/NIAID NIH HHS/United States
- R-01 AI-30048/AI/NIAID NIH HHS/United States
- R-01 AI066232-01/AI/NIAID NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R56 AI043603/AI/NIAID NIH HHS/United States
- R01 AI074862/AI/NIAID NIH HHS/United States
- R37 AI066232/AI/NIAID NIH HHS/United States
- R01 AI043603/AI/NIAID NIH HHS/United States
- R01 AI066232/AI/NIAID NIH HHS/United States
- AI07019/AI/NIAID NIH HHS/United States
- N01 AI030048/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

