Naive mouse macrophages become activated following recognition of L5178Y lymphoma cells via concurrent ligation of CD40, NKG2D, and CD18 molecules
- PMID: 19201847
- PMCID: PMC2757113
- DOI: 10.4049/jimmunol.0800443
Naive mouse macrophages become activated following recognition of L5178Y lymphoma cells via concurrent ligation of CD40, NKG2D, and CD18 molecules
Abstract
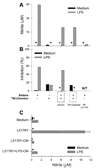
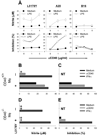
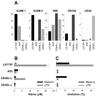
Under different circumstances, tumors can inhibit or activate macrophage (Mphi) effector functions. We studied the mechanisms of tumor-Mphi interactions leading to Mphi activation. The results show that L5178Y mouse T cell lymphoma cells can prime naive mouse Mphi to subsequent LPS stimulation, resulting in increased NO production and antilymphoma effects in vitro. L5178Y cells, but not naive splenocytes, primed Mphi to ligation of TLR4 but not TLR9. L5178Y-primed Mphi incubated with LPS showed down-regulation of CD40 and up-regulation of NKG2D expression. Although L5178Y T cell lymphoma cells primed naive mouse Mphi, several other mouse and human cells lines failed to prime mouse Mphi. Neither L5178Y-conditioned supernatants nor coculture of Mphi and L5178Y cells in Transwells resulted in priming, indicating that direct L5178Y cell-Mphi contact was needed. Several receptor-ligand pairs are reciprocally expressed on Mphi and L5178Y cell membranes and can be potentially involved in Mphi priming. Of these, the CD40-CD154 pair played the most important role, as blocking the interaction of these molecules substantially reduced in vitro Mphi priming. Furthermore, simultaneous blocking of interactions between CD40-CD154, NKG2D-H60, and CD18-ICAM-1/2 led to complete abrogation of Mphi-mediated NO secretion and complete inhibition of Mphi-mediated tumor cell cytostasis. The priming of Mphi to LPS with L5178Y cells was also observed in vivo. These results suggest that contact with certain tumor cells via CD40, NKG2D, and CD18 molecules on the Mphi may facilitate Mphi-mediated antitumor immune surveillance.
Figures








References
-
- Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305:200–205. - PubMed
-
- Chang CC, Ferrone S. NK cell activating ligands on human malignant cells: molecular and functional defects and potential clinical relevance. Semin. Cancer. Biol. 2006;16:383–392. - PubMed
-
- Lamagna C, Aurrand-Lions M, Imhof BA. Dual role of macrophages in tumor growth and angiogenesis. J. Leukoc. Biol. 2006;80:705–713. - PubMed
-
- Greeneltch KM, Schneider M, Steinberg SM, Liewehr DJ, Stewart TJ, Liu K, Abrams SI. Host immunosurveillance controls tumor growth via IFN regulatory factor-8 dependent mechanisms. Cancer. Res. 2007;67:10406–10416. - PubMed
-
- Bottino C, Castriconi R, Moretta L, Moretta A. Cellular ligands of activating NK receptors. Trends Immunol. 2005;26:221–226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

