Allergic airway hyperresponsiveness-enhancing gammadelta T cells develop in normal untreated mice and fail to produce IL-4/13, unlike Th2 and NKT cells
- PMID: 19201853
- PMCID: PMC2688721
- DOI: 10.4049/jimmunol.0803280
Allergic airway hyperresponsiveness-enhancing gammadelta T cells develop in normal untreated mice and fail to produce IL-4/13, unlike Th2 and NKT cells
Abstract
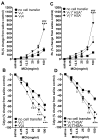
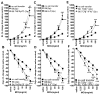
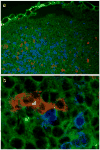
Allergic airway hyperresponsiveness (AHR) in OVA-sensitized and challenged mice, mediated by allergen-specific Th2 cells and Th2-like invariant NKT (iNKT) cells, develops under the influence of enhancing and inhibitory gammadelta T cells. The AHR-enhancing cells belong to the Vgamma1(+) gammadelta T cell subset, cells that are capable of increasing IL-5 and IL-13 levels in the airways in a manner like Th2 cells. They also synergize with iNKT cells in mediating AHR. However, unlike Th2 cells, the AHR enhancers arise in untreated mice, and we show here that they exhibit their functional bias already as thymocytes, at an HSA(high) maturational stage. In further contrast to Th2 cells and also unlike iNKT cells, they could not be stimulated to produce IL-4 and IL-13, consistent with their synergistic dependence on iNKT cells in mediating AHR. Mice deficient in IFN-gamma, TNFRp75, or IL-4 did not produce these AHR-enhancing gammadelta T cells, but in the absence of IFN-gamma, spontaneous development of these cells was restored by adoptive transfer of IFN-gamma-competent dendritic cells from untreated donors. The i.p. injection of OVA/aluminum hydroxide restored development of the AHR enhancers in all of the mutant strains, indicating that the enhancers still can be induced when they fail to develop spontaneously, and that they themselves need not express TNFRp75, IFN-gamma, or IL-4 to exert their function. We conclude that both the development and the cytokine potential of the AHR-enhancing gammadelta T cells differs critically from that of Th2 cells and NKT cells, despite similar influences of these cell populations on AHR.
Figures
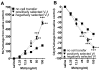



Similar articles
-
Plasticity of invariant NKT cell regulation of allergic airway disease is dependent on IFN-gamma production.J Immunol. 2010 Jul 1;185(1):253-62. doi: 10.4049/jimmunol.0902301. Epub 2010 Jun 4. J Immunol. 2010. PMID: 20525882
-
Different potentials of gamma delta T cell subsets in regulating airway responsiveness: V gamma 1+ cells, but not V gamma 4+ cells, promote airway hyperreactivity, Th2 cytokines, and airway inflammation.J Immunol. 2004 Mar 1;172(5):2894-902. doi: 10.4049/jimmunol.172.5.2894. J Immunol. 2004. PMID: 14978091
-
Vgamma1+ T cells and tumor necrosis factor-alpha in ozone-induced airway hyperresponsiveness.Am J Respir Cell Mol Biol. 2009 Apr;40(4):454-63. doi: 10.1165/rcmb.2008-0346OC. Epub 2008 Oct 16. Am J Respir Cell Mol Biol. 2009. PMID: 18927346 Free PMC article.
-
Adjuvant-dependent regulation of interleukin-17 expressing γδ T cells and inhibition of Th2 responses in allergic airways disease.Respir Res. 2014 Aug 14;15(1):90. doi: 10.1186/s12931-014-0090-5. Respir Res. 2014. PMID: 25123451 Free PMC article.
-
iNKT cells in allergic disease.Curr Top Microbiol Immunol. 2007;314:269-91. doi: 10.1007/978-3-540-69511-0_11. Curr Top Microbiol Immunol. 2007. PMID: 17593665 Review.
Cited by
-
The role of the γ δ T cell in allergic diseases.J Immunol Res. 2014;2014:963484. doi: 10.1155/2014/963484. Epub 2014 Jun 3. J Immunol Res. 2014. PMID: 24995350 Free PMC article. Review.
-
Diversity of γδ T-cell antigens.Cell Mol Immunol. 2013 Jan;10(1):13-20. doi: 10.1038/cmi.2012.45. Epub 2012 Oct 22. Cell Mol Immunol. 2013. PMID: 23085946 Free PMC article. Review.
-
A dual nature of γδ T cell immune memory responses.Elife. 2025 Jul 23;14:e104887. doi: 10.7554/eLife.104887. Elife. 2025. PMID: 40700036 Free PMC article. Review.
-
γδ T Lymphocytes in Asthma: a Complicated Picture.Arch Immunol Ther Exp (Warsz). 2021 Mar 4;69(1):4. doi: 10.1007/s00005-021-00608-7. Arch Immunol Ther Exp (Warsz). 2021. PMID: 33661375 Free PMC article. Review.
-
Role of γδ T Cells in Lung Inflammation.Open Immunol J. 2009;2:143-150. doi: 10.2174/1874226200902010143. Epub 2009 Oct 23. Open Immunol J. 2009. PMID: 26550059 Free PMC article.
References
-
- Steinman L. A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T cell-mediated tissue damage. Nature Medicine. 2007;13:139–145. - PubMed
-
- Else KJ, Finkelman FD. Intestinal nematode parasites, cytokines and effector mechanisms. Int J Parasitol. 1998;28:145–1158. - PubMed
-
- Ramalingam TR, Reiman RM, Wynn TA. Exploiting worm and allergy models to understand Th2 cytokine biology. Current Opinion in Allergy and Clinical Immunology. 2005;5:392–398. - PubMed
-
- Li L, Lee HH, Bell JJ, Gregg RK, Ellis JS, Gessner A, Zaghouani H. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity. 2004;20:429–440. - PubMed
-
- Kaiko GE, Phipps S, Hickey DK, Lam CE, Hansbro PM, Foster PS, Beagley KW. Chlamydia muridarum infection subverts dendritic cell function to promote Th2 immunity and airways hyperreactivity. J Immunol. 2008;180:2225–2232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

