TANK-binding kinase-1 plays an important role during in vitro and in vivo type I IFN responses to DNA virus infections
- PMID: 19201879
- PMCID: PMC2656676
- DOI: 10.4049/jimmunol.0802466
TANK-binding kinase-1 plays an important role during in vitro and in vivo type I IFN responses to DNA virus infections
Abstract
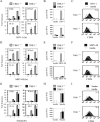
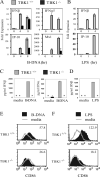
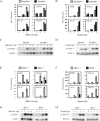
TANK-binding kinase-1 (TBK1) and the inducible IkappaB kinase (IKK-i) have recently been shown to activate type I IFN responses elicited by intracellular detection of RNA or DNA from infecting viruses. Detection of viral RNA is mediated by retinoic acid inducible gene-I or melanoma differentiation-associated gene-5 pathways in which TBK1 and IKK-i have been demonstrated to play redundant roles in IFN activation. In this study, we have examined whether such redundancy occurs in the type I IFN response to DNA viral challenges by examining induction of IFNs and IFN-mediated signaling and gene programs in TBK1(-/-) macrophages. In contrast to the normal IFN responses in TBK1(-/-) macrophages infected with an RNA virus, IFN responses were severely abrogated during DNA virus infections in TBK1(-/-) macrophages. Because both TBK1 and IKK-i are expressed in macrophages, our studies suggest that TBK1 and IKK-i differ functionally in DNA virus-mediated IFN responses; however, they are redundant in RNA virus-mediated IFN responses. Confirmatively, reconstitution of TBK1(-/-)IKK-i(-/-) fibroblasts revealed that TBK1 rescued IFN responses to transfected B-DNA to a much stronger degree than IKK-i. Finally, we demonstrate the requirement for the TBK1-IFN regulatory factor-3 pathway in host defense against a DNA virus infection in vivo.
Figures
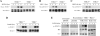

References
-
- Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I Interferons (alpha/beta) in Immunity and Autoimmunity. Annu Rev Immunol. 2005;23:307–336. - PubMed
-
- Malmgaard L. Induction and regulation of IFNs during viral infections. J Interferon Cytokine Res. 2004;24:439–454. - PubMed
-
- Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. - PubMed
-
- Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

