TNFalpha-dependent hepatic steatosis and liver degeneration caused by mutation of zebrafish S-adenosylhomocysteine hydrolase
- PMID: 19201949
- PMCID: PMC2685951
- DOI: 10.1242/dev.027565
TNFalpha-dependent hepatic steatosis and liver degeneration caused by mutation of zebrafish S-adenosylhomocysteine hydrolase
Abstract
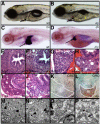
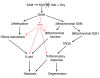
Hepatic steatosis and liver degeneration are prominent features of the zebrafish ducttrip (dtp) mutant phenotype. Positional cloning identified a causative mutation in the gene encoding S-adenosylhomocysteine hydrolase (Ahcy). Reduced Ahcy activity in dtp mutants led to elevated levels of S-adenosylhomocysteine (SAH) and, to a lesser degree, of its metabolic precursor S-adenosylmethionine (SAM). Elevated SAH in dtp larvae was associated with mitochondrial defects and increased expression of tnfa and pparg, an ortholog of the mammalian lipogenic gene. Antisense knockdown of tnfa rescued hepatic steatosis and liver degeneration in dtp larvae, whereas the overexpression of tnfa and the hepatic phenotype were unchanged in dtp larvae reared under germ-free conditions. These data identify an essential role for tnfa in the mutant phenotype and suggest a direct link between SAH-induced methylation defects and TNF expression in human liver disorders associated with elevated TNFalpha. Although heterozygous dtp larvae had no discernible phenotype, hepatic steatosis was present in heterozygous adult dtp fish and in wild-type adult fish treated with an Ahcy inhibitor. These data argue that AHCY polymorphisms and AHCY inhibitors, which have shown promise in treating autoimmunity and other disorders, may be a risk factor for steatosis, particularly in patients with diabetes, obesity and liver disorders such as hepatitis C infection. Supporting this idea, hepatic injury and steatosis have been noted in patients with recently discovered AHCY mutations.
Figures


 P<0.05; NS, not
significant (P=0.3). (D) Calculated SAM:SAH ratio for the four
conditions in C. Differences in the SAM:SAH ratio between B and D arise from
experimental variation. (E) Anti-methylcytosine (αMeC) slot blot
of genomic DNA derived from wild-type or dtp larvae, with Methylene
Blue counterstain (MeBl). (F) Western blot showing reduced levels of
methylated lysine residues (αMeK) in protein from dtp larvae;
actin provided a loading control (αactin).
P<0.05; NS, not
significant (P=0.3). (D) Calculated SAM:SAH ratio for the four
conditions in C. Differences in the SAM:SAH ratio between B and D arise from
experimental variation. (E) Anti-methylcytosine (αMeC) slot blot
of genomic DNA derived from wild-type or dtp larvae, with Methylene
Blue counterstain (MeBl). (F) Western blot showing reduced levels of
methylated lysine residues (αMeK) in protein from dtp larvae;
actin provided a loading control (αactin).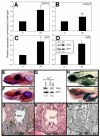


References
-
- Adinolfi, L. E., Ingrosso, D., Cesaro, G., Cimmino, A., D'Anto, M., Capasso, R., Zappia, V. and Ruggiero, G. (2005). Hyperhomocysteinemia and the MTHFR C677T polymorphism promote steatosis and fibrosis in chronic hepatitis C patients. Hepatology 41, 995-1003. - PubMed
-
- Agger, K., Cloos, P. A., Christensen, J., Pasini, D., Rose, S., Rappsilber, J., Issaeva, I., Canaani, E., Salcini, A. E. and Helin, K. (2007). UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature 449, 731-734. - PubMed
-
- Agrimi, G., Di Noia, M. A., Marobbio, C. M., Fiermonte, G., Lasorsa, F. M. and Palmieri, F. (2004). Identification of the human mitochondrial S-adenosylmethionine transporter: bacterial expression, reconstitution, functional characterization and tissue distribution. Biochem. J. 379, 183-190. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

