Catalytic improvement and evolution of atrazine chlorohydrolase
- PMID: 19201959
- PMCID: PMC2663207
- DOI: 10.1128/AEM.02634-08
Catalytic improvement and evolution of atrazine chlorohydrolase
Abstract
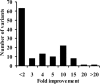
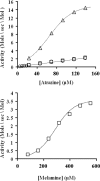
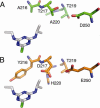
The atrazine chlorohydrolase AtzA has evolved within the past 50 years to catalyze the hydrolytic dechlorination of the herbicide atrazine. It is of wide research interest for two reasons: first, catalytic improvement of the enzyme would facilitate its application in bioremediation, and second, because of its recent evolution, it presents a rare opportunity to examine the early stages in the acquisition of new catalytic activities. Using a structural model of the AtzA-atrazine complex, a region of the substrate-binding pocket was targeted for combinatorial randomization. Identification of improved variants through this process informed the construction of a variant AtzA enzyme with 20-fold improvement in its k(cat)/K(m) value compared with that of the wild-type enzyme. The reduction in K(m) observed in the AtzA variants has allowed the full kinetic profile for the AtzA-catalyzed dechlorination of atrazine to be determined for the first time, revealing the hitherto-unreported substrate cooperativity in AtzA. Since substrate cooperativity is common among deaminases, which are the closest structural homologs of AtzA, it is possible that this phenomenon is a remnant of the catalytic activity of the evolutionary progenitor of AtzA. A catalytic mechanism that suggests a plausible mechanistic route for the evolution of dechlorinase activity in AtzA from an ancestral deaminase is proposed.
Figures

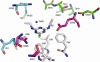
References
-
- Adair, G. S. 1925. The hemoglobin system. VI. The oxygen dissociation curve of hemoglobin. J. Biol. Chem. 63:529-545.
-
- Alcalde, M., M. Ferrer, F. J. Plou, and A. Ballesteros. 2006. Environmental biocatalysis: from remediation with enzymes to novel green processes. Trends Biotechnol. 24:281-287. - PubMed
-
- Arnold, K., L. Bordoli, J. Kopp, and T. Schwede. 2006. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195-201. - PubMed
-
- Bell, A. M., and N. C. Duke. 2005. Effects of Photosystem II inhibiting herbicides on mangroves—preliminary toxicology trials. Mar. Pollut. Bull. 51:297-307. - PubMed
-
- Belluck, D. A., S. L. Benjamin, and T. Dawson. 1991. Groundwater contamination by atrazine and its metabolites—risk assessment, policy, and legal implications. ACS Symp. Ser. 459:254-273.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

