Ser67Asp and His68Asp substitutions in candida parapsilosis carbonyl reductase alter the coenzyme specificity and enantioselectivity of ketone reduction
- PMID: 19201968
- PMCID: PMC2663231
- DOI: 10.1128/AEM.02519-08
Ser67Asp and His68Asp substitutions in candida parapsilosis carbonyl reductase alter the coenzyme specificity and enantioselectivity of ketone reduction
Abstract
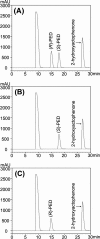
A short-chain carbonyl reductase (SCR) from Candida parapsilosis catalyzes an anti-Prelog reduction of 2-hydroxyacetophenone to (S)-1-phenyl-1,2-ethanediol (PED) and exhibits coenzyme specificity for NADPH over NADH. By using site-directed mutagenesis, the mutants were designed with different combinations of Ser67Asp, His68Asp, and Pro69Asp substitutions inside or adjacent to the coenzyme binding pocket. All mutations caused a significant shift of enantioselectivity toward the (R)-configuration during 2-hydroxyacetophenone reduction. The S67D/H68D mutant produced (R)-PED with high optical purity and yield in the NADH-linked reaction. By kinetic analysis, the S67D/H68D mutant resulted in a nearly 10-fold increase and a 20-fold decrease in the k(cat)/K(m) value when NADH and NADPH were used as the cofactors, respectively, but maintaining a k(cat) value essentially the same with respect to wild-type SCR. The ratio of K(d) (dissociation constant) values between NADH and NADPH for the S67D/H68D mutant and SCR were 0.28 and 1.9 respectively, which indicates that the S67D/H68D mutant has a stronger preference for NADH and weaker binding for NADPH. Moreover, the S67D/H68D enzyme exhibited a secondary structure and melting temperature similar to the wild-type form. It was also found that NADH provided maximal protection against thermal and urea denaturation for S67D/H68D, in contrast to the effective protection by NADP(H) for the wild-type enzyme. Thus, the double point mutation S67D/H68D successfully converted the coenzyme specificity of SCR from NADP(H) to NAD(H) as well as the product enantioselectivity without disturbing enzyme stability. This work provides a protein engineering approach to modify the coenzyme specificity and enantioselectivity of ketone reduction for short-chain reductases.
Figures

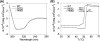
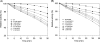

References
-
- Arnold, P., S. Tam, L. Yan, M. E. Baker, F. J. Frey, and A. Odermatt. 2003. Glutamate-115 renders specificity of human 11β-hydroxysteroid dehydrogenase type 2 for the cofactor NAD+. Mol. Cell. Endocrinol. 201:177-187. - PubMed
-
- Bocanegra, J. A., N. S. Scrutton, and R. N. Perham. 1993. Creation of an NADP-dependent pyruvate dehydrogenase multienzyme complex by protein engineering. Biochemistry 32:2737-2740. - PubMed
-
- Fisher, M., J. T. Kroon, W. Martindale, A. R. Stuitje, A. R. Slabas, and J. B. Rafferty. 2000. The X-ray structure of Brassica napus beta-keto acyl carrier protein reductase and its implications for substrate binding and catalysis. Structure 8:339-347. - PubMed
-
- Ghosh, D., V. Z. Pletnev, D. W. Zhu, Z. Wawrzak, W. L. Duax, W. Pangborn, F. Labrie, and S. X. Lin. 1995. Structure of human estrogenic 17 β-hydroxysteroid dehydrogenase at 2.20 Å resolution. Structure 3:503-513. - PubMed
-
- Ghosh, D., M. Sawicki, V. Pletnev, M. Erman, S. Ohno, S. Nakajin, and W. L. Duax. 2001. Porcine carbonyl reductase. structural basis for a functional monomer in short chain dehydrogenases/reductases. J. Biol. Chem. 276:18457-18463. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

