Desensitization of beta-adrenergic receptors in lung injury induced by 2-chloroethyl ethyl sulfide, a mustard analog
- PMID: 19202564
- PMCID: PMC2863039
- DOI: 10.1002/jbt.20265
Desensitization of beta-adrenergic receptors in lung injury induced by 2-chloroethyl ethyl sulfide, a mustard analog
Abstract
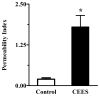
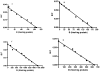
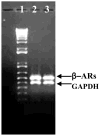
2-Choloroethyl Ethyl Sulfide (CEES) exposure causes inflammatory lung diseases, including acute respiratory distress syndrome (ARDS) and pulmonary fibrosis. This may be associated with oxidative stress, which has been implicated in the desensitization of beta-adrenergic receptors (beta-ARs). The objective of this study was to investigate whether lung injury induced by intratracheal CEES exposure (2 mg/kg body weight) causes desensitization of beta-ARs. The animals were sacrificed after 7 days and lungs were removed. Lung injury was established by measuring the leakage of iodinated-bovine serum albumin ([(125)I]-BSA) into lung tissue. Receptor-binding characteristics were determined by measuring the binding of [(3)H] dihydroalprenolol ([(3)H] DHA) (0.5-24 nM) to membrane fraction in the presence and absence of DLDL-propranolol (10 micro M). Both high- and low-affinity beta-ARs were identified in the lung. Binding capacity was significantly higher in low-affinity site in both control and experimental groups. Although CEES exposure did not change K(D) and B(max) at the high-affinity site, it significantly decreased both K(D) and B(max) at low affinity sites. A 20% decrease in beta(2)-AR mRNA level and a 60% decrease in membrane protein levels were observed in the experimental group. Furthermore, there was significantly less stimulation of adenylate cyclase activity by both cholera toxin and isoproterenol in the experimental group in comparison to the control group. Treatment of lungs with 3-isobutyl-1-methylxanthine (IBMX), an inhibitor of phosphodiesterase (PDE) could not abolish the difference between the control group and the experimental group on the stimulation of the adenylate cyclase activity. Thus, our study indicates that CEES-induced lung injury is associated with desensitization of beta(2)-AR.
(c) 2009 Wiley Periodicals, Inc.
Figures
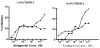


Similar articles
-
Inhibition of cholinephosphotransferase activity in lung injury induced by 2-chloroethyl ethyl sulfide, a mustard analog.J Biochem Mol Toxicol. 2005;19(5):289-97. doi: 10.1002/jbt.20092. J Biochem Mol Toxicol. 2005. PMID: 16292752
-
Effects of intratracheal exposure of 2-chloroethyl ethyl sulfide (CEES) on the activation of CCAAT-enhancer-binding protein (C/EBP) and its protection by antioxidant liposome.J Biochem Mol Toxicol. 2017 May;31(5):10.1002/jbt.21882. doi: 10.1002/jbt.21882. Epub 2016 Nov 30. J Biochem Mol Toxicol. 2017. PMID: 27900814 Free PMC article.
-
Modulation of the expression of superoxide dismutase gene in lung injury by 2-chloroethyl ethyl sulfide, a mustard analog.J Biochem Mol Toxicol. 2006;20(3):142-9. doi: 10.1002/jbt.20128. J Biochem Mol Toxicol. 2006. PMID: 16788954
-
Prophylactic protection by N-acetylcysteine against the pulmonary injury induced by 2-chloroethyl ethyl sulfide, a mustard analogue.J Biochem Mol Toxicol. 2003;17(3):177-84. doi: 10.1002/jbt.10076. J Biochem Mol Toxicol. 2003. PMID: 12815614
-
Ontogeny of fetal adenylate cyclase; mechanisms for regulation of beta-adrenergic receptors.J Dev Physiol. 1989 Nov;12(5):249-61. J Dev Physiol. 1989. PMID: 2699325 Review.
Cited by
-
Countermeasures against Pulmonary Threat Agents.J Pharmacol Exp Ther. 2024 Jan 17;388(2):560-567. doi: 10.1124/jpet.123.001822. J Pharmacol Exp Ther. 2024. PMID: 37863486 Free PMC article. Review.
-
Silymarin/curcumin loaded albumin nanoparticles coated by chitosan as muco-inhalable delivery system observing anti-inflammatory and anti COVID-19 characterizations in oleic acid triggered lung injury and in vitro COVID-19 experiment.Int J Biol Macromol. 2022 Feb 15;198:101-110. doi: 10.1016/j.ijbiomac.2021.12.073. Epub 2021 Dec 28. Int J Biol Macromol. 2022. PMID: 34968533 Free PMC article.
-
Effect of dobutamine on lung aquaporin 5 in endotoxine shock-induced acute lung injury rabbit.J Thorac Dis. 2015 Aug;7(8):1467-77. doi: 10.3978/j.issn.2072-1439.2015.08.22. J Thorac Dis. 2015. PMID: 26380773 Free PMC article.
-
Sulfur mustard-induced pulmonary injury: therapeutic approaches to mitigating toxicity.Pulm Pharmacol Ther. 2011 Feb;24(1):92-9. doi: 10.1016/j.pupt.2010.09.004. Epub 2010 Sep 17. Pulm Pharmacol Ther. 2011. PMID: 20851203 Free PMC article. Review.
-
Stem Cell-Based Therapies and Tissue Engineering of Trachea as Promising Therapeutic Methods in Mustard Gas Exposed Patients.Int J Organ Transplant Med. 2018;9(4):145-154. Epub 2018 Nov 1. Int J Organ Transplant Med. 2018. PMID: 30863517 Free PMC article. Review.
References
-
- Smith MG, Stone W, Crawford K, Ward P, Till GO, Das SK. A promising new treatment for mustard gas with the potential to substantially reduce the threat posed by chemical, biological and radiological agents. Jane’s Chem-Bio Web. 2003:1–5.
-
- Papirmeister B, Fenster AJ, Robinson SI, Ford RD. Sulfur mustard injury: Description of lesions and resulting incapacitations. In: Fenster AJ, editor. Medical defense against mustard gas. Toxic mechanisms and pharmacological implications. Boca Raton, FL: CRC; 1991. pp. 13–42.
-
- Worwser U. Toxicology of mustard gas. Trends Pharmacol Sci. 1991;12:164–167. - PubMed
-
- Momeni AZ, Enshaelh S, Meghdadi SM, Amindjavaheri M. Skin manifestation of mustard gas. Clinical study of 535 patients exposed to mustard gas. Arch Dermatol. 1992;128:775–780. - PubMed
-
- Calvet JH, Jarrean PH, Levame M, D’ortho MP, Lorino H, Harf A, Mavier IM. Acute and chronic respiratory effects of sulfur mustard intoxication in guinea pigs. J Appl Physiol. 1994;76:681–688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

