Intra-operative intra-peritoneal chemotherapy with cisplatin in patients with peritoneal carcinomatosis of ovarian cancer
- PMID: 19203351
- PMCID: PMC2644300
- DOI: 10.1186/1477-7819-7-14
Intra-operative intra-peritoneal chemotherapy with cisplatin in patients with peritoneal carcinomatosis of ovarian cancer
Abstract
Background: Intra-peritoneal (i.p.) chemotherapy is an encouraging treatment option for ovarian cancer with peritoneum involvement in addition with intravenous (i.v.) chemotherapy. Intra-operative i.p. chemotherapy is an interesting method of administration by enhancing the diffusion of chemotherapy. This study had assessed the feasibility of intra-operative i.p. chemotherapy in patients with peritoneal carcinoma of ovarian cancer.
Methods: From January 2003 to February 2006, 47 patients with stage III ovarian cancer were treated with standard paclitaxel carboplatin intravenous chemotherapy and debulking surgery with intra-operative i.p. chemotherapy. After optimal cytoreductive surgery, defined by no unresectable residual disease > 1 cm, i.p. chemotherapy was performed during surgery. The peritoneal cavity was filled by 3 litres of isotonic saline pre-heated at 37 degrees and 90 mg of cisplatin. The sequence was repeated twice during 2 hours based on previous published studies which optimized the cisplatin dosage and exposure duration. Optimal diffusion was obtained by stirring by hands during the 2 hours.
Results: Median age was 59.6 years. No severe haematological or non-haematological toxicity induced by intra operative i.p. chemotherapy was reported. No patient died due to the complications of surgery or the i.p. chemotherapy. No neurotoxicity occurred, and one patients had renal impairment.
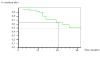
Conclusion: This study demonstrates the feasibility of intra-operative i.p. chemotherapy with cisplatin after optimal resection of peritoneal tumor nodules. Further randomized trials are planned to investigate the clinical benefit of this therapeutic modality.
Figures
Similar articles
-
Paclitaxel/carboplatin versus cyclophosphamide/carboplatin in peritoneal carcinomatosis of the ovary.Eur J Gynaecol Oncol. 2004;25(2):195-6. Eur J Gynaecol Oncol. 2004. PMID: 15032280 Clinical Trial.
-
Patterns of recurrence in advanced epithelial ovarian, fallopian tube and peritoneal cancers treated with intraperitoneal chemotherapy.Gynecol Oncol. 2012 Oct;127(1):51-4. doi: 10.1016/j.ygyno.2012.05.026. Epub 2012 May 30. Gynecol Oncol. 2012. PMID: 22659193
-
First-line intraperitoneal cisplatin-paclitaxel and intravenous ifosfamide in Stage IIIc ovarian epithelial cancer.Eur J Gynaecol Oncol. 2004;25(3):327-32. Eur J Gynaecol Oncol. 2004. PMID: 15171311 Clinical Trial.
-
The use of PIPAC (pressurized intraperitoneal aerosol chemotherapy) in gynecological oncology: a statement by the "Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR)", the Swiss and Austrian AGO, and the North-Eastern German Society of Gynaecologic Oncology.Arch Gynecol Obstet. 2018 Apr;297(4):837-846. doi: 10.1007/s00404-018-4673-0. Epub 2018 Jan 22. Arch Gynecol Obstet. 2018. PMID: 29356953 Review.
-
[Malignant epithelial ovarian cancer: Role of intra peritoneal chemotherapy and hyperthermic intra peritoneal chemotherapy (HIPEC): Article drafted from the French Guidelines in oncology entitled "Initial management of patients with epithelial ovarian cancer" developed by FRANCOGYN, CNGOF, SFOG, GINECO-ARCAGY under the aegis of CNGOF and endorsed by INCa].Gynecol Obstet Fertil Senol. 2019 Feb;47(2):214-221. doi: 10.1016/j.gofs.2019.01.001. Epub 2019 Feb 1. Gynecol Obstet Fertil Senol. 2019. PMID: 30712963 Review. French.
Cited by
-
Investigation of the effectiveness of hyperthermic intraperitoneal chemotherapy in experimental colorectal peritoneal metastasis model.Pleura Peritoneum. 2023 May 10;8(3):123-131. doi: 10.1515/pp-2023-0002. eCollection 2023 Sep. Pleura Peritoneum. 2023. PMID: 37662606 Free PMC article.
-
Intraperitoneal chemotherapy in ovarian cancer: a review of tolerance and efficacy.Cancer Manag Res. 2012;4:413-22. doi: 10.2147/CMAR.S31070. Epub 2012 Nov 23. Cancer Manag Res. 2012. PMID: 23226073 Free PMC article.
-
Cytoreductive surgery (SRC) and hyperthermic intraperitoneal chemotherapy (HIPEC) for treatment of peritoneal carcinomatosis: Our initial experience and technical details.Ulus Cerrahi Derg. 2015 Sep 1;31(3):138-47. doi: 10.5152/UCD.2015.2990. eCollection 2015. Ulus Cerrahi Derg. 2015. PMID: 26504417 Free PMC article.
-
Incorporation of paclitaxel-based hyperthermic intraperitoneal chemotherapy in patients with advanced-stage ovarian cancer treated with neoadjuvant chemotherapy followed by interval debulking surgery: a protocol-based pilot study.J Gynecol Oncol. 2019 Jan;30(1):e3. doi: 10.3802/jgo.2019.30.e3. Epub 2018 Sep 10. J Gynecol Oncol. 2019. PMID: 30479087 Free PMC article.
-
Intraoperative hyperthermic intraperitoneal chemotherapy after cytoreductive surgery in ovarian cancer peritoneal carcinomatosis: systematic review of current results.J Cancer Res Clin Oncol. 2009 Dec;135(12):1637-45. doi: 10.1007/s00432-009-0667-4. Epub 2009 Aug 23. J Cancer Res Clin Oncol. 2009. PMID: 19701772 Free PMC article.
References
-
- Ozols RF. Update on the management of ovarian cancer. Cancer J. 2002;8:S22–30. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical