Cathepsin D expression level affects alpha-synuclein processing, aggregation, and toxicity in vivo
- PMID: 19203374
- PMCID: PMC2644690
- DOI: 10.1186/1756-6606-2-5
Cathepsin D expression level affects alpha-synuclein processing, aggregation, and toxicity in vivo
Abstract
Background: Elevated SNCA gene expression and intracellular accumulation of the encoded alpha-synuclein (aSyn) protein are associated with the development of Parkinson disease (PD). To date, few enzymes have been examined for their ability to degrade aSyn. Here, we explore the effects of CTSD gene expression, which encodes the lysosomal protease cathepsin D (CathD), on aSyn processing.
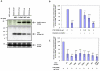
Results: Over-expression of human CTSD cDNA in dopaminergic MES23.5 cell cultures induced the marked proteolysis of exogenously expressed aSyn proteins in a dose-dependent manner. Unexpectedly, brain extractions, Western blotting and ELISA quantification revealed evidence for reduced levels of soluble endogenous aSyn in ctsd knock-out mice. However, these CathD-deficient mice also contained elevated levels of insoluble, oligomeric aSyn species, as detected by formic acid extraction. In accordance, immunohistochemical studies of ctsd-mutant brain from mice, sheep and humans revealed selective synucleinopathy-like changes that varied slightly among the three species. These changes included intracellular aSyn accumulation and formation of ubiquitin-positive inclusions. Furthermore, using an established Drosophila model of human synucleinopathy, we observed markedly enhanced retinal toxicity in ctsd-null flies.
Conclusion: We conclude from these complementary investigations that: one, CathD can effectively degrade excess aSyn in dopaminergic cells; two, ctsd gene mutations result in a lysosomal storage disorder that includes microscopic and biochemical evidence of aSyn misprocessing; and three, CathD deficiency facilitates aSyn toxicity. We therefore postulate that CathD promotes 'synucleinase' activity, and that enhancing its function may lower aSyn concentrations in vivo.
Figures






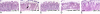
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

