Roles of transient receptor potential channels in pain
- PMID: 19203589
- PMCID: PMC2683630
- DOI: 10.1016/j.brainresrev.2008.12.018
Roles of transient receptor potential channels in pain
Abstract
Pain perception begins with the activation of primary sensory nociceptors. Over the past decade, flourishing research has revealed that members of the Transient Receptor Potential (TRP) ion channel family are fundamental molecules that detect noxious stimuli and transduce a diverse range of physical and chemical energy into action potentials in somatosensory nociceptors. Here we highlight the roles of TRP vanilloid 1 (TRPV1), TRP melastatin 8 (TRPM8) and TRP ankyrin 1 (TRPA1) in the activation of nociceptors by heat and cold environmental stimuli, mechanical force, and by chemicals including exogenous plant and environmental compounds as well as endogenous inflammatory molecules. The contribution of these channels to pain and somatosensation is discussed at levels ranging from whole animal behavior to molecular modulation by intracellular signaling proteins. An emerging theme is that TRP channels are not simple ion channel transducers of one or two stimuli, but instead serve multidimensional roles in signaling sensory stimuli that are exceptionally diverse in modality and in their environmental milieu.
Figures



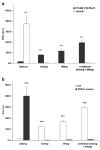
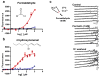









References
-
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

