Complementary and alternative medicine for the allergist-immunologist: where do I start?
- PMID: 19203654
- PMCID: PMC3891367
- DOI: 10.1016/j.jaci.2009.01.001
Complementary and alternative medicine for the allergist-immunologist: where do I start?
Abstract
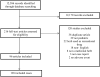
Complementary and alternative medicine (CAM) therapies present a growing information management challenge for physicians because nearly 40% of their patients may be using and another 50% may be considering use of CAM as part of their healthcare regimen. The National Health Statistics Reports for 2007 described the most commonly used nonvitamin, nonmineral therapy as natural products (eg, herbals at 17.7%). More than 5% of children under the age of 18 years used CAM for allergic conditions including asthma. The amount and quality of information available and concerns about liability risk represent a challenge for most physicians. This review focuses on considerations for approaching a CAM-related consultation, incorporating legal and logistic factors affecting how such an encounter should be approached. A 10-step process is presented that addresses different components of CAM consultations and what should be documented. Access to timely, high-quality information regarding product specific efficacy and safety data, as found in the Natural Medicines Comprehensive Database, is needed to support CAM consultation efficiently. Understanding of serious adverse events associated with CAM is limited; an international need exists for improved safety surveillance and information sharing. Allergy-immunology, as a specialty with expertise in adverse drug reaction evaluation and management, has a unique opportunity to support enhanced CAM-related adverse events evaluations, reporting, and research.
Conflict of interest statement
Conflicts of Interest: There are no conflicts of interest to report.
Figures
Comment in
-
Role of complementary and alternative medicine in the field of allergy and clinical immunology.J Allergy Clin Immunol. 2009 Feb;123(2):317-8. doi: 10.1016/j.jaci.2008.12.1112. J Allergy Clin Immunol. 2009. PMID: 19203655 No abstract available.
References
-
- Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United states, 2007. Natl Health Stat Report. 2008;(12):1–23. - PubMed
-
- U.S. Food and Drug Administration. Dietary supplement and nonprescription drug consumer protection act. 2007
-
- Kelly WN, Arellano FM, Barnes J, et al. Guidelines for submitting adverse event reports for publication. Drug Saf. 2007;30(5):367–373. - PubMed
-
- Begg C, Cho M, Eastwood S, et al. Improving the quality of reporting of randomized controlled trials. the CONSORT statement. JAMA. 1996;276(8):637–639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical