Activation of neurokinin 3 receptor increases Na(v)1.9 current in enteric neurons
- PMID: 19204045
- PMCID: PMC2678219
- DOI: 10.1113/jphysiol.2009.169409
Activation of neurokinin 3 receptor increases Na(v)1.9 current in enteric neurons
Abstract
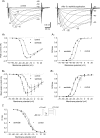
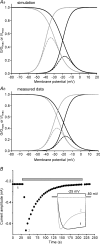
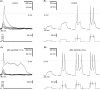
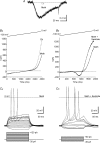
The intrinsic primary afferent neurons (IPANs) of the guinea pig enteric nervous system express Na(v)1.9 sodium channels that produce a persistent TTX-resistant current having a low activation threshold and slow gating kinetics. These neurons receive slow EPSPs induced mainly by the activation of neurokinin 3 receptors (NK3r). Here, we demonstrate that senktide, a specific NK3r agonist, potentiates the Na(v)1.9 current (I(Nav1.9)) in IPANs. Using whole-cell patch-clamp recordings from IPANs in duodenum longitudinal muscle/myenteric plexus preparations, we show that short (1-5 s) and long (up to 1 min) applications of senktide, increase the I(Nav1.9) peak current up to 13-fold. The effect, blocked by a NK3r antagonist SB235375 is transient, lasting approximately 2 min and is due to a negative shift of the activation voltage by approximately 20 mV and of fast inactivation by approximately 10 mV. As a consequence, the window current resulting from the product of the activation and fast inactivation curves is shifted and enlarged. The transient effect of senktide is likely to be due to the fast desensitization of NK3r. Protein kinase C (PKC) activation with phorbol or oleoyl acetylglycerol also increases I(Nav1.9), although persistently, by inducing similar voltage-dependent changes. Current-clamp experiments showed that I(Nav1.9) modulation by senktide lowers action potential threshold and increases excitability. The increase in I(Nav1.9) by NK3r activation is also likely to amplify slow EPSPs generated in the IPANs. These changes in excitability potentially have a profound effect on the entire enteric synaptic circuit and ultimately on gut motility and secretion.
Figures






Comment in
-
Na(V)-igating excitement in the enteric nervous system.J Physiol. 2009 Apr 1;587(Pt 7):1377. doi: 10.1113/jphysiol.2009.170852. J Physiol. 2009. PMID: 19336607 Free PMC article. No abstract available.
References
-
- Alex G, Kunze WA, Furness JB, Clerc N. Comparison of the effects of neurokinin-3 receptor blockade on two forms of slow synaptic transmission in myenteric AH neurons. Neuroscience. 2001;104:263–269. - PubMed
-
- Bertrand PP, Galligan JJ. Signal-transduction pathways causing slow synaptic excitation in guinea pig myenteric AH neurons. Am J Physiol Gastrointest Liver Physiol. 1995;269:G710–720. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

