{beta} subunit-specific modulations of BK channel function by a mutation associated with epilepsy and dyskinesia
- PMID: 19204046
- PMCID: PMC2678220
- DOI: 10.1113/jphysiol.2009.169243
{beta} subunit-specific modulations of BK channel function by a mutation associated with epilepsy and dyskinesia
Abstract
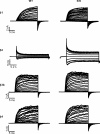
Large conductance Ca(2+)-activated K(+) (BK) channels modulate many physiological processes including neuronal excitability, synaptic transmission and regulation of myogenic tone. A gain-of-function (E/D) mutation in the pore-forming alpha subunit (Slo1) of the BK channel was recently identified and is linked to human neurological diseases of coexistent generalized epilepsy and paroxysmal dyskinesia. Here we performed macroscopic current recordings to examine the effects of the E/D mutation on the gating kinetics, and voltage and Ca(2+) dependence of the BK channel activation in the presence of four different beta subunits (beta1-4). These beta subunits are expressed in a tissue-specific pattern and modulate BK channel function differently, providing diversity and specificity for BK channels in various physiological processes. Our results show that in human (h) Slo1-only channels, the E/D mutation increased the rate of opening and decreased the rate of closing, allowing a greater number of channels to open at more negative potentials both in the presence and absence of Ca(2+) due to increased Ca(2+) affinity and enhanced activation compared with the wild-type channels. Even in the presence of beta subunits, the E/D mutation exhibited these changes with the exception of beta3b, where Ca(2+) sensitivity changed little. However, quantitative examination of these changes shows the diversity of each beta subunit and the differential modulation of these subunits by the E/D mutation. For example, in the presence of the beta1 subunit the E/D mutation increased Ca(2+) sensitivity less but enhanced channel activation in the absence of Ca(2+) more than in hSlo1-only channels, while in the presence of the beta2 subunit the E/D mutation also altered inactivation properties. These findings suggest that depending on the distribution of the various beta subunits in the brain, the E/D mutation can modulate BK channels differently to contribute to the pathophysiology of epilepsy and dyskinesia. Additionally, these results also have implications on physiological processes in tissues other than the brain where BK channels play an important role.
Figures
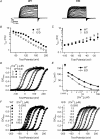
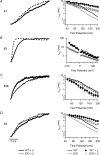





 where base is the minimum value of I/I0, a is the I/I0 (max) −I/I0 (min), V1/2 is the half I/I0 voltage and rate is slope of the curve.
where base is the minimum value of I/I0, a is the I/I0 (max) −I/I0 (min), V1/2 is the half I/I0 voltage and rate is slope of the curve.
References
-
- Adelman JP, Shen KZ, Kavanaugh MP, Warren RA, Wu YN, Lagrutta A, Bond CT, North RA. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992;9:209–216. - PubMed
-
- Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991;253:551–555. - PubMed
-
- Behrens R, Nolting A, Reimann F, Schwarz M, Waldschutz R, Pongs O. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel β subunit family. FEBS Lett. 2000;474:99–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

