CFTR functions as a bicarbonate channel in pancreatic duct cells
- PMID: 19204187
- PMCID: PMC2654087
- DOI: 10.1085/jgp.200810122
CFTR functions as a bicarbonate channel in pancreatic duct cells
Abstract
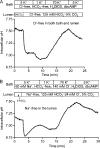
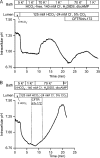
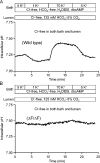
Pancreatic duct epithelium secretes a HCO(3)(-)-rich fluid by a mechanism dependent on cystic fibrosis transmembrane conductance regulator (CFTR) in the apical membrane. However, the exact role of CFTR remains unclear. One possibility is that the HCO(3)(-) permeability of CFTR provides a pathway for apical HCO(3)(-) efflux during maximal secretion. We have therefore attempted to measure electrodiffusive fluxes of HCO(3)(-) induced by changes in membrane potential across the apical membrane of interlobular ducts isolated from the guinea pig pancreas. This was done by recording the changes in intracellular pH (pH(i)) that occurred in luminally perfused ducts when membrane potential was altered by manipulation of bath K(+) concentration. Apical HCO(3)(-) fluxes activated by cyclic AMP were independent of Cl(-) and luminal Na(+), and substantially inhibited by the CFTR blocker, CFTR(inh)-172. Furthermore, comparable HCO(3)(-) fluxes observed in ducts isolated from wild-type mice were absent in ducts from cystic fibrosis (Delta F) mice. To estimate the HCO(3)(-) permeability of the apical membrane under physiological conditions, guinea pig ducts were luminally perfused with a solution containing 125 mM HCO(3)(-) and 24 mM Cl(-) in the presence of 5% CO(2). From the changes in pH(i), membrane potential, and buffering capacity, the flux and electrochemical gradient of HCO(3)(-) across the apical membrane were determined and used to calculate the HCO(3)(-) permeability. Our estimate of approximately 0.1 microm sec(-1) for the apical HCO(3)(-) permeability of guinea pig duct cells under these conditions is close to the value required to account for observed rates of HCO(3)(-) secretion. This suggests that CFTR functions as a HCO(3)(-) channel in pancreatic duct cells, and that it provides a significant pathway for HCO(3)(-) transport across the apical membrane.
Figures



References
-
- Argent B.E., Arkle S., Cullen M.J., Green R. 1986. Morphological, biochemical and secretory studies on rat pancreatic ducts maintained in tissue culture.Q. J. Exp. Physiol. 71:633–648 - PubMed
-
- Argent B.E., Gray M.A., Steward M.C., Case R.M. 2006. Cell physiology of pancreatic ducts. In Physiology of the Gastrointestinal Tract, fourth edition Johnson L.R., Barrett K.E., Ghishan F.K., Merchant J.L., Said H.M., Wood J.D., editors Elsevier Academic Press, New York: 1371–1396
-
- Arkle S., Lee C.M., Cullen M.J., Argent B.E. 1986. Isolation of ducts from the pancreas of copper-deficient rats.Q. J. Exp. Physiol. 71:249–265 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

