Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034
- PMID: 19204207
- PMCID: PMC2667826
- DOI: 10.1200/JCO.2008.17.5984
Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034
Abstract
Purpose: Approximately 50% of glioblastomas (GBMs) are characterized by overexpression of the epidermal growth factor receptor (EGFR) and EGFR gene amplification. In approximately 25% of instances, constitutively activated EGFR mutants are present. These observations make EGFR-inhibiting drugs a logical approach for trials in recurrent GBM.
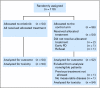
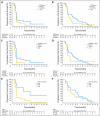
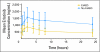
Patients and methods: In a randomized, controlled, phase II trial, 110 patients with progressive GBM after prior radiotherapy were randomly assigned to either erlotinib or a control arm that received treatment with either temozolomide or carmustine (BCNU). The primary end point was 6-month progression-free survival (PFS). Tumor specimens obtained at first surgery were investigated for EGFR expression; EGFRvIII mutants; EGFR amplification; EGFR mutations in exons 18, 19, and 21; and pAkt. These results were correlated with outcome. Pharmacokinetic analysis was part of the study. RESULTS; Treatment was well tolerated in general; skin toxicity was the most frequent adverse effect of erlotinib. The 6-month PFS rate in the erlotinib arm was 11.4% (95% CI, 4.6% to 21.5%), and it was 24% in the control arm. Of all explored biomarkers, only low pAkt expression appeared to be of borderline significance to an improved outcome. None of the eight patients who had tumors with EGFRvIII mutant presence and PTEN expression had 6-month PFS. The use of enzyme-inducing anticonvulsants significantly increased erlotinib clearance, but pharmacokinetic findings were not related to outcome.
Conclusion: Erlotinib has insufficient single-agent activity in unselected GBM. No clear biomarker associated with improved outcome to erlotinib was identified.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Frederick L, Wang XY, Eley G, et al. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60:1383–1387. - PubMed
-
- Haas-Kogan DA, Prados MD, Tihan T, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97:880–887. - PubMed
-
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

