Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis
- PMID: 19204730
- PMCID: PMC2785034
- DOI: 10.1038/nature07713
Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis
Erratum in
- Nature. 2010 Jun 17;465(7300):966
Abstract
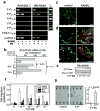
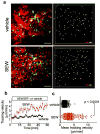
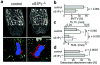
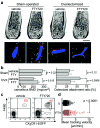
Osteoclasts are the only somatic cells with bone-resorbing capacity and, as such, they have a critical role not only in normal bone homeostasis (called 'bone remodelling') but also in the pathogenesis of bone destructive disorders such as rheumatoid arthritis and osteoporosis. A major focus of research in the field has been on gene regulation by osteoclastogenic cytokines such as receptor activator of NF-kappaB-ligand (RANKL, also known as TNFSF11) and TNF-alpha, both of which have been well documented to contribute to osteoclast terminal differentiation. A crucial process that has been less well studied is the trafficking of osteoclast precursors to and from the bone surface, where they undergo cell fusion to form the fully differentiated multinucleated cells that mediate bone resorption. Here we report that sphingosine-1-phosphate (S1P), a lipid mediator enriched in blood, induces chemotaxis and regulates the migration of osteoclast precursors not only in culture but also in vivo, contributing to the dynamic control of bone mineral homeostasis. Cells with the properties of osteoclast precursors express functional S1P(1) receptors and exhibit positive chemotaxis along an S1P gradient in vitro. Intravital two-photon imaging of bone tissues showed that a potent S1P(1) agonist, SEW2871, stimulated motility of osteoclast precursor-containing monocytoid populations in vivo. Osteoclast/monocyte (CD11b, also known as ITGAM) lineage-specific conditional S1P(1) knockout mice showed osteoporotic changes due to increased osteoclast attachment to the bone surface. Furthermore, treatment with the S1P(1) agonist FTY720 relieved ovariectomy-induced osteoporosis in mice by reducing the number of mature osteoclasts attached to the bone surface. Together, these data provide evidence that S1P controls the migratory behaviour of osteoclast precursors, dynamically regulating bone mineral homeostasis, and identifies a critical control point in osteoclastogenesis that may have potential as a therapeutic target.
Figures
References
-
- Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. - PubMed
-
- Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–649. - PubMed
-
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. - PubMed
-
- Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–570. - PubMed
-
- Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

