Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay
- PMID: 19204785
- PMCID: PMC2635847
Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay
Abstract
Purpose: To examine the ability of retinal pigment epithelial (RPE) cells derived from human embryonic stem cells (HESC) to phagocytose photoreceptor outer segments, and to determine whether exposure to human retina induces any morphological changes in these cells.
Methods: HESC-RPE cells were derived from a super-confluent preparation of the Shef1 HESC line. Pigmented colonies were isolated and expanded into pigmented monolayers on Matrigel matrix-coated dishes or filters. Cells were exposed to fluorescently labeled outer segments isolated from the porcine eye and assessed for phagocytic activity at regular intervals. Expression of molecules associated with RPE phagocytosis was analyzed by RT-PCR, immunocytochemistry, and western blot. The role of Mer Tyrosine Kinase (MERTK) in the phagocytosis of outer segments was investigated using antibodies directed against MERTK to block function. In a novel approach, cells were also exposed to fresh human neural retina tissue then examined by electron microscopy for evidence of phagocytosis and changes in cell morphology.
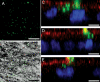
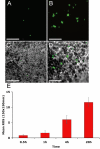
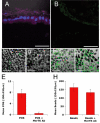
Results: HESC-derived RPE cells are capable of phagocytosing isolated porcine outer segments and express molecules associated with RPE-specific phagocytosis, including MERTK. Pre-incubation with antibodies against MERTK blocked phagocytosis of photoreceptor outer segments, but not polystyrene beads. HESC-RPE cells also phagocytosed outer segments in a novel human retinal explant system. Furthermore co-culture adjacent to human retina tissue in this preparation resulted in the appearance of features in HESC-derived RPE cells normally observed only as the RPE matures.
Conclusions: The ingestion of photoreceptor outer segments from an isolated population and an artificial ex vivo human retina system demonstrates HESC-derived RPE cells are functional. HESC-derived RPE possess the relevant molecules required for phagocytosis, including MERTK, which is essential for the phagocytosis of outer segments but not latex beads. Furthermore, some changes observed in cell morphology after co-culture with human retina may have implications for understanding the full development and differentiation of RPE cells.
Figures



References
-
- Klimanskaya I, Hipp J, Rezai KA, West M, Atala A, Lanza R. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells. 2004;6:217–45. - PubMed
-
- Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, Akaike A, Sasai Y, Takahashi M. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26:215–24. - PubMed
-
- Lund RD, Wang S, Klimanskaya I, Holmes T, Ramos-Kelsey R, Lu B, Girman S, Bischoff N, Sauvé Y, Lanza R. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells. 2006;8:189–99. - PubMed
-
- Vugler A, Carr AJ, Lawrence J, Chen LL, Burrell K, Wright A, Lundh P, Semo M, Ahmado A, Gias C, da Cruz L, Moore H, Andrews P, Walsh J, Coffey P. Elucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation. Exp Neurol. 2008;214:347–61. - PubMed
-
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
