Electrostatic and functional analysis of the seven-bladed WD beta-propellers
- PMID: 19204818
- PMCID: PMC2614187
- DOI: 10.4137/ebo.s743
Electrostatic and functional analysis of the seven-bladed WD beta-propellers
Abstract
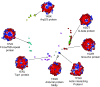
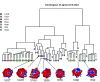
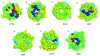
beta-propeller domains composed of WD repeats are highly ubiquitous and typically used as multi-site docking platforms to coordinate and integrate the activities of groups of proteins. Here, we have used extensive homology modelling of the WD40-repeat family of seven-bladed beta-propellers coupled with subsequent structural classification and clustering of these models to define subfamilies of beta-propellers with common structural, and probable, functional characteristics. We show that it is possible to assign seven-bladed WD beta-propeller proteins into functionally different groups based on the information gained from homology modelling. We examine general structural diversity within the WD40-repeat family of seven-bladed beta-propellers and demonstrate that seven-bladed beta-propellers composed of WD-repeats are structurally distinct from other seven-bladed beta-propellers. We further provide some insights into the multifunctional diversity of the seven-bladed WD beta-propeller surfaces. This report once again reinforces the importance of structural data and the usefulness of homology models in functional classification.
Keywords: WD protein; electrostatics; evolutionary trace; protein clustering; β-propeller.
Figures
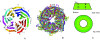


References
-
- Blomberg N, Gabdoulline RR, et al. Classification of protein sequences by homology modeling and quantitative analysis of electrostatic similarity. Proteins. 1999;37:379–87. - PubMed
-
- Blomberg N, Nilges M. Functional diversity of PH domains: an exhaustive modelling study. Fold Des. 1997;2:343–55. - PubMed
-
- Botti SA, Felder CE, et al. Electrotactins: a class of adhesion proteins with conserved electrostatic and structural motifs. Protein Eng. 1998;11:415–20. - PubMed
-
- Chakravarty S, Sanchez R. Systematic analysis of added-value in simple comparative models of protein structure. Structure. 2004;12:1461–70. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

