Induction of muscle weakness by local inflammation: an experimental animal model
- PMID: 19205846
- PMCID: PMC2790866
- DOI: 10.1007/s00011-008-8093-7
Induction of muscle weakness by local inflammation: an experimental animal model
Abstract
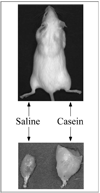
Objective and design: The objective of this study was to characterize the response of skeletal muscle to a localized inflammation induced by the inflammatory agent casein.
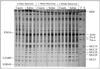
Methods: An inflammatory agent, casein, was injected into the right hindlimb and saline was injected into the left hindlimb of normal adult mice, once daily for six consecutive days. Inflammatory response was monitored by immunohistochemical labeling of leukocytes. Muscle protein levels were determined by electrophoresis and muscle function was determined by isometric force measurements.
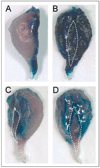
Results: Local inflammation was induced by casein in association with the accumulation of extensive neutrophils and macrophages in the soleus muscle. This local inflammation resulted in a shift in myosin heavy chain (MHC) isoform expression and a significant reduction in total MHC concentration in the soleus. Maximal twitch and tetanic forces were significantly reduced in the inflamed soleus. Contractile function in soleus was fully restored after two weeks of recovery, along with the restoration of protein concentration and the disappearance of inflammatory cells.
Conclusion: This study establishes a unique and robust model in which mechanisms of local inflammation induced muscle protein degradation, reduction of contractile force, and subsequent recovery from this condition can be further studied.
Figures







References
-
- Verleye M, Heulard I, Stephens JR, Levy RH, Gillardin JM. Effects of citrulline malate on bacterial lipopolysaccharide induced endotoxemia in rats. Arzneimittelforschung. 1995;45:712–715. - PubMed
-
- Walsmith J, Roubenoff R. Cachexia in rheumatoid arthritis. Int J Cardiol. 2002;85:89–99. - PubMed
-
- Muscaritoli M, Bossola M, Aversa Z, Bellantone R, Rossi Fanelli F. Prevention and treatment of cancer cachexia: new insights into an old problem. Eur J Cancer. 2006;42:31–41. - PubMed
-
- Coats AJ. Origin of symptoms in patients with cachexia with special reference to weakness and shortness of breath. Int J Cardiol. 2002;85:133–139. - PubMed
-
- Sharma R, Anker SD. Cytokines, apoptosis and cachexia: the potential for TNF antagonism. Int J Cardiol. 2002;85:161–171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

