Improved accuracy of 15N-1H scalar and residual dipolar couplings from gradient-enhanced IPAP-HSQC experiments on protonated proteins
- PMID: 19205898
- PMCID: PMC2753394
- DOI: 10.1007/s10858-009-9299-x
Improved accuracy of 15N-1H scalar and residual dipolar couplings from gradient-enhanced IPAP-HSQC experiments on protonated proteins
Abstract
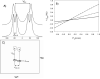
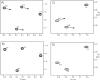
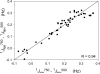
The presence of dipole-dipole cross-correlated relaxation as well as unresolved E.COSY effects adversely impacts the accuracy of (1)J(NH) splittings measured from gradient-enhanced IPAP-HSQC spectra. For isotropic samples, the size of the systematic errors caused by these effects depends on the values of (2)J(NHalpha), (3)J(NHbeta) and (3)J(HNHalpha). Insertion of band-selective (1)H decoupling pulses in the IPAP-HSQC experiment eliminates these systematic errors and for the protein GB3 yields (1)J(NH) splittings that agree to within a root-mean-square difference of 0.04 Hz with values measured for perdeuterated GB3. Accuracy of the method is also highlighted by a good fit to the GB3 structure of the (1)H-(15)N RDCs extracted from the minute differences in (1)J(NH) splitting measured at 500 and 750 MHz (1)H frequencies, resulting from magnetic susceptibility anisotropy. A nearly complete set of (2)J(NHalpha) couplings was measured in GB3 in order to evaluate whether the impact of cross-correlated relaxation is dominated by the (15)N-(1)H(alpha) or (15)N-(1)H(beta) dipolar interaction. As expected, we find that (2)J(NHalpha) < or = 2 Hz, with values in the alpha-helix (0.86 +/- 0.52 Hz) slightly larger than in beta-sheet (0.66 +/- 0.26 Hz). Results indicate that under isotropic conditions, N-H(N)/N-H(beta) cross-correlated relaxation often dominates. Unresolved E.COSY effects under isotropic conditions involve (3)J(HNHalpha) and J(NHalpha), but when weakly aligned any aliphatic proton proximate to both N and H(N) can contribute.
Figures
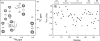

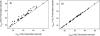
Similar articles
-
Facile measurement of ¹H-¹5N residual dipolar couplings in larger perdeuterated proteins.J Biomol NMR. 2010 Oct;48(2):65-70. doi: 10.1007/s10858-010-9441-9. Epub 2010 Aug 7. J Biomol NMR. 2010. PMID: 20694505 Free PMC article.
-
Accurate measurement of (3)J(HNHα) couplings in small or disordered proteins from WATERGATE-optimized TROSY spectra.J Biomol NMR. 2016 Jan;64(1):1-7. doi: 10.1007/s10858-015-0004-y. Epub 2015 Dec 10. J Biomol NMR. 2016. PMID: 26660434 Free PMC article.
-
Measurement of eight scalar and dipolar couplings for methine-methylene pairs in proteins and nucleic acids.J Biomol NMR. 2005 Mar;31(3):201-16. doi: 10.1007/s10858-005-0175-z. J Biomol NMR. 2005. PMID: 15803394
-
Interference between cross-correlated relaxation and the measurement of scalar and dipolar couplings by Quantitative J.J Biomol NMR. 2006 May;35(1):1-16. doi: 10.1007/s10858-006-0028-4. Epub 2006 May 19. J Biomol NMR. 2006. PMID: 16791736
-
Homonuclear decoupling for enhancing resolution and sensitivity in NOE and RDC measurements of peptides and proteins.J Magn Reson. 2014 Apr;241:97-102. doi: 10.1016/j.jmr.2013.11.006. Epub 2013 Nov 22. J Magn Reson. 2014. PMID: 24360766 Free PMC article. Review.
Cited by
-
NMR solution structures of Runella slithyformis RNA 2'-phosphotransferase Tpt1 provide insights into NAD+ binding and specificity.Nucleic Acids Res. 2021 Sep 27;49(17):9607-9624. doi: 10.1093/nar/gkab241. Nucleic Acids Res. 2021. PMID: 33880546 Free PMC article.
-
Solution Structure of the Carboxy-Terminal Tandem Repeat Domain of Eukaryotic Elongation Factor 2 Kinase and Its Role in Substrate Recognition.J Mol Biol. 2019 Jul 12;431(15):2700-2717. doi: 10.1016/j.jmb.2019.05.019. Epub 2019 May 18. J Mol Biol. 2019. PMID: 31108082 Free PMC article.
-
Structure of the competence pilus major pilin ComGC in Streptococcus pneumoniae.J Biol Chem. 2017 Aug 25;292(34):14134-14146. doi: 10.1074/jbc.M117.787671. Epub 2017 Jun 28. J Biol Chem. 2017. PMID: 28659339 Free PMC article.
-
Aha1 Exhibits Distinctive Dynamics Behavior and Chaperone-Like Activity.Molecules. 2021 Mar 30;26(7):1943. doi: 10.3390/molecules26071943. Molecules. 2021. PMID: 33808352 Free PMC article.
-
Dissecting C-H∙∙∙π and N-H∙∙∙π Interactions in Two Proteins Using a Combined Experimental and Computational Approach.Sci Rep. 2019 Dec 27;9(1):20149. doi: 10.1038/s41598-019-56607-4. Sci Rep. 2019. PMID: 31882834 Free PMC article.
References
-
- Archer SJ, Ikura M, Torchia DA, Bax A. An alternative 3D NMR technique for correlating backbone 15N with sidechain Hβ resonances in larger proteins. J. Magn. Reson. 1991;95:636–641.
-
- Bouvignies G, Markwick P, Brueschweiler R, Blackledge M. Simultaneous determination of protein backbone structure and dynamics from residual dipolar couplings. J. Am. Chem. Soc. 2006;128:15100–15101. - PubMed
-
- Bruschweiler R. New approaches to the dynamic interpretation and prediction of NMR relaxation data from proteins. Curr. Opin. Struct. Biol. 2003;13:175–183. - PubMed
-
- Bystrov VF. Spin-spin couplings and the conformational states of peptide systems. Prog. NMR Spectrosc. 1976;10:41–81.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

