Improved accuracy of 15N-1H scalar and residual dipolar couplings from gradient-enhanced IPAP-HSQC experiments on protonated proteins
- PMID: 19205898
- PMCID: PMC2753394
- DOI: 10.1007/s10858-009-9299-x
Improved accuracy of 15N-1H scalar and residual dipolar couplings from gradient-enhanced IPAP-HSQC experiments on protonated proteins
Abstract
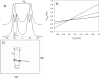
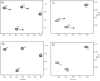
The presence of dipole-dipole cross-correlated relaxation as well as unresolved E.COSY effects adversely impacts the accuracy of (1)J(NH) splittings measured from gradient-enhanced IPAP-HSQC spectra. For isotropic samples, the size of the systematic errors caused by these effects depends on the values of (2)J(NHalpha), (3)J(NHbeta) and (3)J(HNHalpha). Insertion of band-selective (1)H decoupling pulses in the IPAP-HSQC experiment eliminates these systematic errors and for the protein GB3 yields (1)J(NH) splittings that agree to within a root-mean-square difference of 0.04 Hz with values measured for perdeuterated GB3. Accuracy of the method is also highlighted by a good fit to the GB3 structure of the (1)H-(15)N RDCs extracted from the minute differences in (1)J(NH) splitting measured at 500 and 750 MHz (1)H frequencies, resulting from magnetic susceptibility anisotropy. A nearly complete set of (2)J(NHalpha) couplings was measured in GB3 in order to evaluate whether the impact of cross-correlated relaxation is dominated by the (15)N-(1)H(alpha) or (15)N-(1)H(beta) dipolar interaction. As expected, we find that (2)J(NHalpha) < or = 2 Hz, with values in the alpha-helix (0.86 +/- 0.52 Hz) slightly larger than in beta-sheet (0.66 +/- 0.26 Hz). Results indicate that under isotropic conditions, N-H(N)/N-H(beta) cross-correlated relaxation often dominates. Unresolved E.COSY effects under isotropic conditions involve (3)J(HNHalpha) and J(NHalpha), but when weakly aligned any aliphatic proton proximate to both N and H(N) can contribute.
Figures
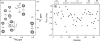

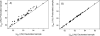
References
-
- Archer SJ, Ikura M, Torchia DA, Bax A. An alternative 3D NMR technique for correlating backbone 15N with sidechain Hβ resonances in larger proteins. J. Magn. Reson. 1991;95:636–641.
-
- Bouvignies G, Markwick P, Brueschweiler R, Blackledge M. Simultaneous determination of protein backbone structure and dynamics from residual dipolar couplings. J. Am. Chem. Soc. 2006;128:15100–15101. - PubMed
-
- Bruschweiler R. New approaches to the dynamic interpretation and prediction of NMR relaxation data from proteins. Curr. Opin. Struct. Biol. 2003;13:175–183. - PubMed
-
- Bystrov VF. Spin-spin couplings and the conformational states of peptide systems. Prog. NMR Spectrosc. 1976;10:41–81.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

