Proteome of synaptosome-associated proteins in spinal cord dorsal horn after peripheral nerve injury
- PMID: 19206110
- PMCID: PMC2768229
- DOI: 10.1002/pmic.200800636
Proteome of synaptosome-associated proteins in spinal cord dorsal horn after peripheral nerve injury
Abstract
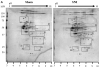
Peripheral nerve injury may lead to neuroadaptive changes of cellular signals in spinal cord that are thought to contribute to central mechanisms underlying neuropathic pain. Here we used a 2-DE-based proteomic technique to determine the global expression changes of synaptosome-associated proteins in spinal cord dorsal horn after unilateral fifth spinal nerve injury (SNI). The fifth lumbar dorsal horns ipsilateral to SNI or sham surgery were harvested on day 14 post-surgery, and the total soluble and synaptosomal fractions were isolated. The proteins derived from the synaptosomal fraction were resolved by 2-DE. We identified 27 proteins that displayed different expression levels after SNI, including proteins involved in transmission and modulation of noxious information, cellular metabolism, membrane receptor trafficking, oxidative stress, apoptosis, and degeneration. Six of the 27 proteins were chosen randomly and further validated in the synaptosomal fraction by Western blot analysis. Unexpectedly, Western blot analysis showed that only one protein in the total soluble fraction exhibited a significant expression change after SNI. The data indicate that peripheral nerve injury changes not only protein expression but also protein subcellular distribution in dorsal horn cells. These changes might participate in the central mechanism that underlies the maintenance of neuropathic pain.
Figures
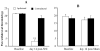


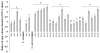
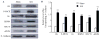
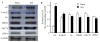
References
-
- Woolf CJ, Salter MW. In: Wall and Melzack's Textbook of Pain. McMahon S, Koltzenburg M, editors. Elsevier; London: 2006. pp. 91–105.
-
- Yang L, Zhang FX, Huang F, Lu YJ, et al. Peripheral nerve injury induces trans-synaptic modification of channels, receptors and signal pathways in rat dorsal spinal cord. Eur J Neurosci. 2004;19:871–883. - PubMed
-
- Coyle DE. Spinal cord transcriptional profile analysis reveals protein trafficking and RNA processing as prominent processes regulated by tactile allodynia. Neuroscience. 2007;144:144–56. - PubMed
-
- Rodriguez PJ, Korostynski M, Kaminska-Chowaniec D, Obara I, et al. Comparison of gene expression profiles in neuropathic and inflammatory pain. J Physiol Pharmacol. 2006;57:401–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

