Age-related decrease in proteasome expression contributes to defective nuclear factor-kappaB activation during hepatic ischemia/reperfusion
- PMID: 19206148
- PMCID: PMC2695826
- DOI: 10.1002/hep.22840
Age-related decrease in proteasome expression contributes to defective nuclear factor-kappaB activation during hepatic ischemia/reperfusion
Abstract
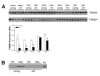
Hepatic ischemia/reperfusion (I/R) leads to liver injury and dysfunction through the initiation of a biphasic inflammatory response that is regulated by the transcription factor nuclear factor kappaB (NF-kappaB). We have previously shown that there is an age-dependent difference in the injury response to hepatic I/R in mice that correlates with divergent activation of NF-kappaB such that young mice have greater NF-kappaB activation, but less injury than old mice. In this study, we investigated the mechanism by which age alters the activation of NF-kappaB in the liver during I/R. Young (4-5 weeks) and old (12-14 months) mice underwent partial hepatic I/R. Livers were obtained for RNA microarray analysis and protein expression assays. Using microarray analysis, we identified age-dependent differences in the expression of genes related to protein ubiquitinylation and the proteasome. In old mice, genes that are involved in the ubiquitin-proteasome pathway were significantly down-regulated during I/R. Consistent with these findings, expression of a critical proteasome subunit, non-adenosine triphosphatase 4 (PSMD4), was reduced in old mice. Expression of the NF-kappaB inhibitory protein, IkappaB alpha, was increased in old mice and was greatly phosphorylated and ubiquitinylated. The data provide strong evidence that the age-related defect in hepatic NF-kappaB signaling during I/R is a result of decreased expression of PSMD4, a proteasome subunit responsible for recognition and recruitment of ubiquitinylated substrates to the proteasome. It appears that decreased PSMD4 expression prevents recruitment of phosphorylated and ubiquitinylated IkappaB alpha to the proteasome, resulting in a defect in NF-kappaB activation.
Figures








Similar articles
-
The peptidyl-prolyl isomerase, Pin1, facilitates NF-kappaB binding in hepatocytes and protects against hepatic ischemia/reperfusion injury.J Hepatol. 2009 Aug;51(2):296-306. doi: 10.1016/j.jhep.2009.04.016. Epub 2009 May 24. J Hepatol. 2009. PMID: 19515451 Free PMC article.
-
Reactive oxygen species mediate liver injury through parenchymal nuclear factor-kappaB inactivation in prolonged ischemia/reperfusion.Am J Pathol. 2009 May;174(5):1776-85. doi: 10.2353/ajpath.2009.080857. Epub 2009 Apr 6. Am J Pathol. 2009. PMID: 19349371 Free PMC article.
-
N-3 PUFA supplementation triggers PPAR-α activation and PPAR-α/NF-κB interaction: anti-inflammatory implications in liver ischemia-reperfusion injury.PLoS One. 2011;6(12):e28502. doi: 10.1371/journal.pone.0028502. Epub 2011 Dec 8. PLoS One. 2011. PMID: 22174823 Free PMC article.
-
Receptor activator of nuclear factor-κB ligand (RANKL) protects against hepatic ischemia/reperfusion injury in mice.Hepatology. 2012 Mar;55(3):888-97. doi: 10.1002/hep.24756. Epub 2012 Jan 13. Hepatology. 2012. PMID: 22031462 Free PMC article.
-
Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity.Annu Rev Immunol. 2000;18:621-63. doi: 10.1146/annurev.immunol.18.1.621. Annu Rev Immunol. 2000. PMID: 10837071 Review.
Cited by
-
miR-219a-5p Ameliorates Hepatic Ischemia/Reperfusion Injury via Impairing TP53BP2.Dig Dis Sci. 2019 Aug;64(8):2177-2186. doi: 10.1007/s10620-019-05535-4. Epub 2019 Feb 22. Dig Dis Sci. 2019. PMID: 30796685
-
Association analyses suggest the effects of RANK and RANKL on age at menarche in Chinese women.Climacteric. 2012 Feb;15(1):75-81. doi: 10.3109/13697137.2011.587556. Epub 2011 Oct 24. Climacteric. 2012. PMID: 22023082 Free PMC article.
-
Protective effects of fish oil, allopurinol, and verapamil on hepatic ischemia-reperfusion injury in rats.J Nat Sci Biol Med. 2015 Jul-Dec;6(2):351-5. doi: 10.4103/0976-9668.160003. J Nat Sci Biol Med. 2015. PMID: 26283828 Free PMC article.
-
Liver Ischemia Reperfusion Injury, Enhanced by Trained Immunity, Is Attenuated in Caspase 1/Caspase 11 Double Gene Knockout Mice.Pathogens. 2020 Oct 24;9(11):879. doi: 10.3390/pathogens9110879. Pathogens. 2020. PMID: 33114395 Free PMC article.
-
Ubiquitin-proteasome system inhibitors and AMPK regulation in hepatic cold ischaemia and reperfusion injury: possible mechanisms.Clin Sci (Lond). 2012 Jul;123(2):93-8. doi: 10.1042/CS20110093. Clin Sci (Lond). 2012. PMID: 22455352 Free PMC article.
References
-
- Hawker F. Liver dysfunction in critical illness. Anaesth Intensive Care. 1991;19:165–181. - PubMed
-
- Huguet C, Gavelli A, Bona S. Hepatic resection with ischemia of the liver exceeding one hour. J Am Coll Surg. 1994;178:454–458. - PubMed
-
- Lemasters JJ, Thurman RG. Reperfusion injury after liver preservation for transplantation. Annu Rev Pharmacol Toxicol. 1997;37:327–338. - PubMed
-
- Liu DL, Jeppsson B, Hakansson CH, Odselius R. Multiple-system organ damage resulting from prolonged hepatic inflow interruption. Arch Surg. 1996;131:442–447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
