Electrophysiology of reactive oxygen production in signaling endosomes
- PMID: 19207039
- PMCID: PMC2872256
- DOI: 10.1089/ars.2008.2448
Electrophysiology of reactive oxygen production in signaling endosomes
Abstract
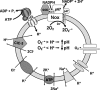
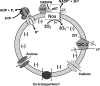
Endosome trafficking and function require acidification by the vacuolar ATPase (V-ATPase). Electrogenic proton (H+) transport reduces the pH and creates a net positive charge in the endosomal lumen. Concomitant chloride (Cl-) influx has been proposed to occur via ClC Cl-=H+ exchangers. This maintains charge balance and drives Cl- accumulation, which may itself be critical to endosome function. Production of reactive oxygen species (ROS) in response to cytokines occurs within specialized endosomes that form in response to receptor occupation. ROS production requires an NADPH oxidase (Nox) and the ClC-3 Cl-=H+ exchanger. Like the V-ATPase, Nox activity is highly electrogenic, but separates charge with an opposite polarity (lumen negative). Here we review established paradigms of early endosomal ion transport focusing on the relation between the V-ATPase and ClC proteins. Electrophysiologic constraints on Nox-mediated vesicular ROS production are then considered. The potential for ClC-3 to participate in charge neutralization of both proton (V-ATPase) and electron (Nox) transport is discussed. It is proposed that uncompensated charge separation generated by Nox enzymatic activity could be used to drive secondary transport into negatively charged vesicles. Further experimentation will be necessary to establish firmly the biochemistry and functional implications of endosomal ROS production.
Figures



Similar articles
-
Direct endosomal acidification by the outwardly rectifying CLC-5 Cl(-)/H(+) exchanger.J Physiol. 2010 Jun 15;588(Pt 12):2033-45. doi: 10.1113/jphysiol.2010.188540. Epub 2010 Apr 26. J Physiol. 2010. PMID: 20421284 Free PMC article.
-
Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins.Nature. 2005 Jul 21;436(7049):424-7. doi: 10.1038/nature03860. Nature. 2005. PMID: 16034422
-
Cl- and H+ coupling properties and subcellular localizations of wildtype and disease-associated variants of the voltage-gated Cl-/H+ exchanger ClC-5.J Biol Chem. 2020 Feb 7;295(6):1464-1473. doi: 10.1074/jbc.RA119.011366. Epub 2019 Dec 18. J Biol Chem. 2020. PMID: 31852738 Free PMC article.
-
The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function.Curr Opin Cell Biol. 2008 Aug;20(4):415-26. doi: 10.1016/j.ceb.2008.03.015. Epub 2008 May 27. Curr Opin Cell Biol. 2008. PMID: 18511251 Free PMC article. Review.
-
Signaling components of redox active endosomes: the redoxosomes.Antioxid Redox Signal. 2009 Jun;11(6):1313-33. doi: 10.1089/ars.2008.2363. Antioxid Redox Signal. 2009. PMID: 19072143 Free PMC article. Review.
Cited by
-
Lipids, lysosomes, and autophagy.J Lipid Res. 2016 Sep;57(9):1619-35. doi: 10.1194/jlr.R067520. Epub 2016 Jun 21. J Lipid Res. 2016. PMID: 27330054 Free PMC article. Review.
-
Chloride channels in stroke.Acta Pharmacol Sin. 2013 Jan;34(1):17-23. doi: 10.1038/aps.2012.140. Epub 2012 Oct 29. Acta Pharmacol Sin. 2013. PMID: 23103617 Free PMC article. Review.
-
Overexpression of SeNHX1 improves both salt tolerance and disease resistance in tobacco.Plant Signal Behav. 2015;10(4):e993240. doi: 10.4161/15592324.2014.993240. Plant Signal Behav. 2015. PMID: 25875967 Free PMC article.
-
The ClC-3 Cl-/H+ antiporter becomes uncoupled at low extracellular pH.J Biol Chem. 2010 Jan 22;285(4):2569-79. doi: 10.1074/jbc.M109.018002. Epub 2009 Nov 19. J Biol Chem. 2010. PMID: 19926787 Free PMC article.
-
Phagosomal proteolysis in dendritic cells is modulated by NADPH oxidase in a pH-independent manner.EMBO J. 2012 Feb 15;31(4):932-44. doi: 10.1038/emboj.2011.440. Epub 2011 Dec 13. EMBO J. 2012. PMID: 22157818 Free PMC article.
References
-
- Accardi A. Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature. 2004;427(6977):803–807. - PubMed
-
- Afanas'ev IB. Competition between superoxide and hydrogen peroxide signaling in heterolytic enzymatic processes. Med Hypotheses. 2006;66(6):1125–1128. - PubMed
-
- Al-Awqati Q. Proton-translocating ATPases. Annu Rev Cell Biol. 1986;2:179–199. - PubMed
-
- Barg S. Huang P. Eliasson L. Nelson DJ. Obermuller S. Rorsman P. Thevenod F. Renstrom E. Priming of insulin granules for exocytosis by granular Cl(−) uptake and acidification. J Cell Sci. 2001;114(Pt 11):2145–2154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

