Immunogenicity of allogeneic adipose-derived stem cells in a rat spinal fusion model
- PMID: 19207041
- PMCID: PMC2746330
- DOI: 10.1089/ten.TEA.2008.0566
Immunogenicity of allogeneic adipose-derived stem cells in a rat spinal fusion model
Abstract
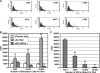
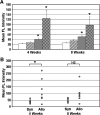
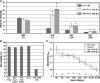
Adipose-derived stem cells (ASCs) express a nonimmunogenic profile as shown by in vitro studies that demonstrate a lack of T cell proliferation to allogeneic ASCs as well as ASC-mediated suppression of mixed lymphocyte reactions. To determine whether these observations would translate in vivo, immune monitoring studies were carried out in conjunction with a rat spinal fusion study. ASCs derived from Fischer or ACI strain rats were loaded onto scaffolds and implanted in Fischer recipients that had undergone the following treatments: (1) No treatment; (2) Scaffold only; (3) Syngeneic ASCs+Scaffold; or (4) Allogeneic ASCs+Scaffold. Half of each group was sacrificed at 4 weeks postimplantation, and the remaining animals were sacrificed at 8 weeks. As determined in a separate study, allogeneic and syngeneic ASCs were equally efficacious in accelerating spinal fusion compared to No treatment and Scaffold only control groups. To determine whether donor ASCs induced an immune response in recipient rats, lymph nodes were harvested for T cell proliferation studies and serum was collected to assess antibody responses. Although T cell priming was not detected to donor alloantigens in recipients at either time point, significant antibody responses were detected to ACI ASCs in animals implanted with syngeneic or allogeneic ASCs. Antibodies were of the IgG isotype, noncytotoxic in the presence of complement, and reactive to fetal bovine serum. These results support the use of allogeneic ASCs for spinal fusion.
Figures



Similar articles
-
Evaluation of cellular and humoral immune responses to allogeneic adipose-derived stem/stromal cells.Methods Mol Biol. 2011;702:133-50. doi: 10.1007/978-1-61737-960-4_11. Methods Mol Biol. 2011. PMID: 21082400
-
Acceleration of spinal fusion using syngeneic and allogeneic adult adipose derived stem cells in a rat model.J Orthop Res. 2009 Mar;27(3):366-73. doi: 10.1002/jor.20735. J Orthop Res. 2009. PMID: 18752292 Free PMC article.
-
Bone regeneration in a canine cranial model using allogeneic adipose derived stem cells and coral scaffold.Biomaterials. 2013 Apr;34(11):2655-64. doi: 10.1016/j.biomaterials.2013.01.004. Epub 2013 Jan 21. Biomaterials. 2013. PMID: 23343633
-
Comparison of Autologous and Allogeneic Adipose-Derived Stem Cells in Kidney Transplantation: Immunological Considerations and Therapeutic Efficacy.J Clin Med. 2024 Sep 27;13(19):5763. doi: 10.3390/jcm13195763. J Clin Med. 2024. PMID: 39407823 Free PMC article. Review.
-
An update on niche composition, signaling and functional regulation of the adipose-derived stem cells.Expert Opin Biol Ther. 2014 Aug;14(8):1091-102. doi: 10.1517/14712598.2014.907785. Epub 2014 Apr 8. Expert Opin Biol Ther. 2014. PMID: 24707959 Review.
Cited by
-
Bone Healing Materials in the Treatment of Recalcitrant Nonunions and Bone Defects.Int J Mol Sci. 2022 Mar 20;23(6):3352. doi: 10.3390/ijms23063352. Int J Mol Sci. 2022. PMID: 35328773 Free PMC article. Review.
-
Understanding the Future Prospects of Synergizing Minimally Invasive Transforaminal Lumbar Interbody Fusion Surgery with Ceramics and Regenerative Cellular Therapies.Int J Mol Sci. 2021 Mar 31;22(7):3638. doi: 10.3390/ijms22073638. Int J Mol Sci. 2021. PMID: 33807361 Free PMC article. Review.
-
Epithelial defect repair in the auricle and auditory meatus by grafting with cultured adipose-derived mesenchymal stem cell aggregate-extracellular matrix.Chin Med J (Engl). 2019 Mar 20;132(6):680-689. doi: 10.1097/CM9.0000000000000125. Chin Med J (Engl). 2019. PMID: 30855349 Free PMC article.
-
Impaired expansion and multipotentiality of adult stromal cells in a rat chronic alcohol abuse model.Alcohol. 2011 Jun;45(4):393-402. doi: 10.1016/j.alcohol.2010.12.005. Epub 2011 Mar 4. Alcohol. 2011. PMID: 21376503 Free PMC article.
-
Fat Grafting: Basic Science, Techniques, and Patient Management.Plast Reconstr Surg Glob Open. 2022 Mar 18;10(3):e3987. doi: 10.1097/GOX.0000000000003987. eCollection 2022 Mar. Plast Reconstr Surg Glob Open. 2022. PMID: 35317456 Free PMC article. Review.
References
-
- Schaffler A. Buchler C. Concise review: adipose tissue-derived stromal cells—basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818. - PubMed
-
- McIntosh K. Zvonic S. Garrett S. Mitchell J.B. Floyd Z.E. Hammill L. Kloster A. Di Halvorsen Y. Ting J.P. Storms R.W. Goh B. Kilroy G. Wu X. Gimble J.M. The immunogenicity of human adipose derived cells: temporal changes in vitro. Stem Cells. 2006;24:1246. - PubMed
-
- Niemeyer P. Kornacker M. Mehlorn A. Seckinger A. Vohrer J. Schmal H. Kasten P. Eckstein V. Südkamp N.P. Krause U. Comparison of immunological properties of bone marrow stromal cells and adipose tissue-derived stem cells before and after osteogenic differentiation in vitro. Tissue Eng. 2007;13:111. - PubMed
-
- Hoogduijn M.J. Crop M.J. Peeters A.M. Van Osch G.J. Balk A.H. Ijzermans J.N. Weimar W. Baan C.C. Human heart, spleen, and perirenal fat-derived mesenchymal stem cells have immunomodulatory capacities. Stem Cells Dev. 2007;16:597. - PubMed
-
- Puissant B. Barreau C. Bourin P. Clavel C. Corre J. Bousquet C. Taureau C. Cousin B. Abbal M. Laharrague P. Penicaud L. Casteilla L. Blancher A. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

