The periosteum as a cellular source for functional tissue engineering
- PMID: 19207046
- PMCID: PMC2792114
- DOI: 10.1089/ten.TEA.2008.0244
The periosteum as a cellular source for functional tissue engineering
Abstract
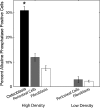
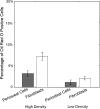
The periosteum, a specialized fibrous tissue composed of fibroblast, osteoblast, and progenitor cells, may be an optimal cell source for tissue engineering based on its accessibility, the ability of periosteal cells to proliferate rapidly both in vivo and in vitro, and the observed differentiation potential of these cells. However, the functional use of periosteum-derived cells as a source for tissue engineering requires an understanding of the ability of such cells to elaborate matrix of different tissues. In this study, we subjected a population of adherent primary periosteum-derived cells to both adipogenic and osteogenic culture conditions. The commitment propensity of periosteal cells was contrasted with that of well-characterized phenotypically pure populations of NIH3T3 fibroblast and MC3T3-E1 osteoblast cell lines. Our results demonstrate that the heterogeneous populations of periosteal cells and NIH3T3 fibroblasts have the ability to express both osteoblast-like and adipocyte-like markers with similar potential. This raises the question of whether fibroblasts within the periosteum may, in fact, have the potential to behave like progenitor cells and play a role in the tissue's multilineage potential or whether there are true stem cells within the periosteum. Further, this study suggests that expanded periosteal cultures may be a source for tissue engineering applications without extensive enrichment or sorting by molecular markers. Thus, this study lays the groundwork for future investigations that will more deeply enumerate the cellular sources and molecular events governing periosteal cell differentiation.
Figures

References
-
- Agata H. Asahina I. Yamazaki Y. Uchida M. Shinohara Y. Honda M.J. Kagami H. Ueda M. Effective bone engineering with periosteum-derived cells. J Dent Res. 2007;86:79. - PubMed
-
- Emans P.J. Surtel D.A. Frings E.J. Bulstra S.K. Kuijer R. In vivo generation of cartilage from periosteum. Tissue Eng. 2005;11:369. - PubMed
-
- Eyckmans J. Luyten F.P. Species specificity of ectopic bone formation using periosteum-derived mesenchymal progenitor cells. Tissue Eng. 2006;12:2203. - PubMed
-
- Hadjiargyrou M. Ahrens W. Rubin C.T. Temporal expression of the chondrogenic and angiogenic growth factor CYR61 during fracture repair. J Bone Miner Res. 2000;15:1014. - PubMed
-
- Hutmacher D.W. Sittinger M. Periosteal cells in bone tissue engineering. Tissue Eng. 2003;9(Suppl 1):S45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

