Pseudomonas aeruginosa Type III secretion system interacts with phagocytes to modulate systemic infection of zebrafish embryos
- PMID: 19207728
- PMCID: PMC2933946
- DOI: 10.1111/j.1462-5822.2009.01288.x
Pseudomonas aeruginosa Type III secretion system interacts with phagocytes to modulate systemic infection of zebrafish embryos
Abstract
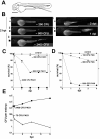
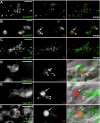
Pseudomonas aeruginosa is an opportunistic human pathogen that can cause serious infection in those with deficient or impaired phagocytes. We have developed the optically transparent and genetically tractable zebrafish embryo as a model for systemic P. aeruginosa infection. Despite lacking adaptive immunity at this developmental stage, zebrafish embryos were highly resistant to P. aeruginosa infection, but as in humans, phagocyte depletion dramatically increased their susceptibility. The virulence of an attenuated P. aeruginosa strain lacking a functional Type III secretion system was restored upon phagocyte depletion, suggesting that this system influences virulence through its effects on phagocytes. Intravital imaging revealed bacterial interactions with multiple blood cell types. Neutrophils and macrophages rapidly phagocytosed and killed P. aeruginosa, suggesting that both cell types play a role in protection against infection. Intravascular aggregation of erythrocytes and other blood cells with resultant circulatory blockage was observed immediately upon infection, which may be relevant to the pathogenesis of thrombotic complications of human P. aeruginosa infections. The real-time visualization capabilities and genetic tractability of the zebrafish infection model should enable elucidation of molecular and cellular details of P. aeruginosa pathogenesis in conditions associated with neutropenia or impaired phagocyte function.
© 2009 Blackwell Publishing Ltd.
Figures
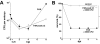


References
-
- Avet-Rochex A, Bergeret E, Attree I, Meister M, Fauvarque MO. Suppression of Drosophila cellular immunity by directed expression of the ExoS toxin GAP domain of Pseudomonas aeruginosa. Cell Microbiol. 2005;7:799–810. - PubMed
-
- Barton GM, Medzhitov R. Toll-like receptors and their ligands. Curr Top Microbiol Immunol. 2002;270:81–92. - PubMed
-
- Bates JM, Mittge E, Kuhlman J, Baden KN, Cheesman SE, Guillemin K. Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev Biol. 2006;297:374–386. - PubMed
-
- Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

