Role of transglutaminase 2 in liver injury via cross-linking and silencing of transcription factor Sp1
- PMID: 19208340
- PMCID: PMC4960455
- DOI: 10.1053/j.gastro.2009.01.007
Role of transglutaminase 2 in liver injury via cross-linking and silencing of transcription factor Sp1
Abstract
Background & aims: Despite high morbidity and mortality of alcoholic liver disease worldwide, the molecular mechanisms underlying alcohol-induced liver cell death are not fully understood. Transglutaminase 2 (TG2) is a cross-linking enzyme implicated in apoptosis. TG2 levels and activity are increased in association with various types of liver injury. However, how TG2 induces hepatic apoptosis is not known.
Methods: Human hepatic cells or primary hepatocytes from rats or TG2+/+ and TG2-/- mice were treated with ethanol. Mice were administered anti-Fas antibody or alcohol. Liver sections were prepared from patients with alcoholic steatohepatitis. Changes in TG2 levels, Sp1 cross-linking and its activities, expression of hepatocyte growth factor receptor, c-Met, and hepatic apoptosis were measured.
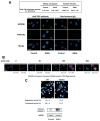
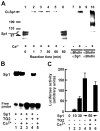
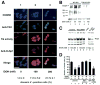
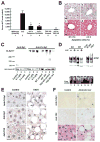
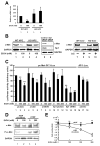
Results: Ethanol induced apoptosis in hepatic cells, enhanced activity and nuclear accumulation of TG2 as well as accumulation of cross-linked and inactivated Sp1, and reduced expression of the Sp1-responsive gene, c-Met. These effects were rescued by TG2 knockdown, restoration of functional Sp1, or addition of hepatocyte growth factor, whereas apoptosis was reproduced by Sp1 knockdown or TG2 overexpression. Compared with TG2+/+ mice, TG2-/- mice showed markedly reduced hepatocyte apoptosis and Sp1 cross-linking following ethanol or anti-Fas treatment. Treatment of TG2+/+ mice with the TG2 inhibitors putrescine or cystamine blocked anti-Fas-induced hepatic apoptosis and Sp1 silencing. Moreover, enhanced expression of cross-linked Sp1 and TG2 was evident in hepatocyte nuclei of patients with alcoholic steatohepatitis.
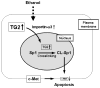
Conclusions: TG2 induces hepatocyte apoptosis via Sp1 cross-linking and inactivation, with resultant inhibition of the expression of c-Met required for hepatic cell viability.
Conflict of interest statement
Conflicts of interest
The authors disclose no conflicts.
Figures
Comment in
-
Transglutaminase cross-links Sp1-mediated transcription to ethanol-induced liver injury.Gastroenterology. 2009 May;136(5):1502-5. doi: 10.1053/j.gastro.2009.03.021. Epub 2009 Mar 21. Gastroenterology. 2009. PMID: 19318103 No abstract available.
References
-
- Ribeiro PS, Cortez-Pinto H, Solá S, et al. Hepatocyte apoptosis, expression of death receptors, and activation of NF-κB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol. 2004;99:1708–1717. - PubMed
-
- Lorand L, Graham RM. Transglutaminases: cross-linking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. - PubMed
-
- Fésüs L, Piacentini M. Transglutaminase 2: an enigmatic enzyme with diverse functions. Trends Biochem Sci. 2002;27:534–539. - PubMed
-
- Mirza A, Liu SL, Frizell E, et al. A role for tissue transglutaminase in hepatic injury and fibrogenesis, and its regulation by NF-kB. Am J Physiol. 1997;272:G281–G288. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

