Intrauterine growth restriction and differential patterns of hepatic growth and expression of IGF1, PCK2, and HSDL1 mRNA in the sheep fetus in late gestation
- PMID: 19208549
- PMCID: PMC2849808
- DOI: 10.1095/biolreprod.108.073569
Intrauterine growth restriction and differential patterns of hepatic growth and expression of IGF1, PCK2, and HSDL1 mRNA in the sheep fetus in late gestation
Abstract
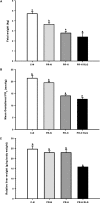
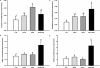
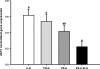
Fetal adaptations to periods of substrate deprivation can result in the programming of glucose intolerance, insulin resistance, and metabolic dysfunction in later life. Placental insufficiency can be associated with either sparing or sacrifice of fetal liver growth, and these different responses may have different metabolic consequences. It is unclear what intrahepatic mechanisms determine the differential responses of the fetal liver to substrate restriction. We investigated the effects of placental restriction (PR) on liver growth and the hepatic expression of SLC2A1, IGF1, IGF2, IGF1R, IGF2R, PPARGC1A, PPARA, PRKAA1, PRKAA2, PCK2, and HSDL1 mRNA in fetal sheep at 140-145 days of gestation. A mean gestational arterial partial pressure of oxygen less than 17 mmHg was defined as hypoxic, and a relative liver of weight more than 2 SD below the mean liver weight of controls was defined as reduced liver growth. Fetuses therefore were defined as control-normoxic (C-N; n = 9), PR-normoxic (PR-N; n = 7), PR-hypoxic (PR-H; n = 8), or PR-hypoxic reduced liver growth (PR-H RLG; n = 4). Hepatic SLC2A1 mRNA expression was highest (P < 0.05) in the PR-H fetuses, in which liver growth was maintained. Expression of IGF1 mRNA was decreased (P < 0.05) only in the PR-H RLG group. Hepatic expression of HSDL1, PPARGC1A, and PCK2 mRNA also were increased (P < 0.05) in the PR-H RLG fetuses. The present study highlights that intrahepatic responses to fetal substrate restriction may exist that protect the liver from decreased growth and, potentially, from a decreased responsiveness to the actions of insulin in postnatal life.
Figures
Similar articles
-
Placental restriction of fetal growth decreases IGF1 and leptin mRNA expression in the perirenal adipose tissue of late gestation fetal sheep.Am J Physiol Regul Integr Comp Physiol. 2008 May;294(5):R1413-9. doi: 10.1152/ajpregu.00787.2007. Epub 2008 Feb 13. Am J Physiol Regul Integr Comp Physiol. 2008. PMID: 18272661
-
Differential regulation of igf1 and igf1r mRNA levels in the two hepatic lobes following intrauterine growth restriction and its treatment with intra-amniotic insulin-like growth factor-1 in ovine fetuses.Reprod Fertil Dev. 2010;22(8):1188-97. doi: 10.1071/RD09292. Reprod Fertil Dev. 2010. PMID: 20883644
-
Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle.Endocrinology. 2009 Jul;150(7):3021-30. doi: 10.1210/en.2008-1789. Epub 2009 Apr 2. Endocrinology. 2009. PMID: 19342452 Free PMC article.
-
Fetal growth restriction and the programming of heart growth and cardiac insulin-like growth factor 2 expression in the lamb.J Physiol. 2011 Oct 1;589(Pt 19):4709-22. doi: 10.1113/jphysiol.2011.211185. Epub 2011 Aug 1. J Physiol. 2011. PMID: 21807611 Free PMC article.
-
Catecholamines mediate multiple fetal adaptations during placental insufficiency that contribute to intrauterine growth restriction: lessons from hyperthermic sheep.J Pregnancy. 2011;2011:740408. doi: 10.1155/2011/740408. Epub 2011 May 11. J Pregnancy. 2011. PMID: 21773031 Free PMC article. Review.
Cited by
-
Fetal growth restriction, catch-up growth and the early origins of insulin resistance and visceral obesity.Pediatr Nephrol. 2010 Apr;25(4):669-77. doi: 10.1007/s00467-009-1407-3. Epub 2009 Dec 22. Pediatr Nephrol. 2010. PMID: 20033220 Review.
-
Identification of candidate genes from androgenic gland in Macrobrachium nipponense regulated by eyestalk ablation.Sci Rep. 2021 Oct 6;11(1):19855. doi: 10.1038/s41598-021-99022-4. Sci Rep. 2021. PMID: 34615913 Free PMC article.
-
Acute-on-chronic: using magnetic resonance imaging to disentangle the haemodynamic responses to acute and chronic fetal hypoxaemia.Front Med (Lausanne). 2024 Jun 12;11:1340012. doi: 10.3389/fmed.2024.1340012. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38933113 Free PMC article.
-
Differential effects of intrauterine growth restriction and a hypersinsulinemic-isoglycemic clamp on metabolic pathways and insulin action in the fetal liver.Am J Physiol Regul Integr Comp Physiol. 2019 May 1;316(5):R427-R440. doi: 10.1152/ajpregu.00359.2018. Epub 2019 Feb 13. Am J Physiol Regul Integr Comp Physiol. 2019. PMID: 30758974 Free PMC article.
-
Chronic anemic hypoxemia increases plasma glucagon and hepatic PCK1 mRNA in late-gestation fetal sheep.Am J Physiol Regul Integr Comp Physiol. 2016 Jul 1;311(1):R200-8. doi: 10.1152/ajpregu.00037.2016. Epub 2016 May 11. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 27170658 Free PMC article.
References
-
- McMillen IC, Robinson JS.Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 2005; 85: 571–633. - PubMed
-
- Hales C, Barker D.Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 1992; 35: 595–601. - PubMed
-
- Simmons RA, Templeton LJ, Gertz SJ.Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes 2001; 50: 2279–2286. - PubMed
-
- Lane RH, MacLennan NK, Hsu JL, Janke SM, Pham TD.Increased hepatic peroxisome proliferator-activated receptor-g coactivator-1 gene expression in a rat model of intrauterine growth retardation and subsequent insulin resistance. Endocrinology 2002; 143: 2486–2490. - PubMed
-
- Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM.Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 2001; 413: 131–138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

