Regulation of facilitative glucose transporters and AKT/MAPK/PRKAA signaling via estradiol and progesterone in the mouse uterine epithelium
- PMID: 19208550
- PMCID: PMC3093992
- DOI: 10.1095/biolreprod.108.072629
Regulation of facilitative glucose transporters and AKT/MAPK/PRKAA signaling via estradiol and progesterone in the mouse uterine epithelium
Abstract
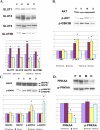
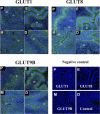
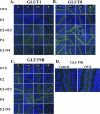
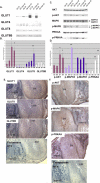
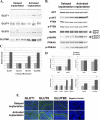
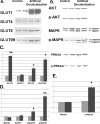
Adequate uterine glucose metabolism is an essential part of embryo implantation and the development of an adequate utero-fetal environment. However, expression of facilitative glucose transporters (GLUTs [solute transporter family SLC2A]) and AKT/MAPK/PRKAA (PRKAA) signaling has not been described in the mouse uterine cells, to our knowledge. The objective of this study was to determine the hormonal regulation of SLC2A protein expression and AKT/MAPK/PRKAA signaling in the mouse uterine epithelial cells during estrous cycles and peri-implantation periods. SLC2As 1, 4, 8, and 9B were highly expressed in the luminal and glandular epithelia of estrous stage. In metestrous and diestrous stages, expression of SLC2As 1, 4, 8, and 9B was lower than that in proestrous stage. Levels of activated phospho-AKT (p-AKT), p-MAPK3, and p-MAPK1 also varied during the estrous cycle. Estrogen and progesterone injection in an ovariectomized mouse (delayed implantation model) resulted in a decrease and an increase, respectively, in expression of GLUTs in the luminal epithelial cells of the uterus. The expression of SLC2A1, SLC2A8, SLC2A9B, p-AKT, p-MAPK3/1, and p-PRKAA was increased in the decidual region of the implantation sites and was significantly increased in the uterus of activated implantation. Using an artificial decidualization mouse model, it was also demonstrated that expression of the same GLUTs, p-MAPK3/1, and p-PRKAA was dramatically higher in the decidualized uteri than that in the control uteri. These results suggest that steroid hormones regulate expression of uterine epithelial GLUTs possibly through AKT/MAPK/PRKAA signaling pathways and that glucose utilization may have an important role in decidualization and possibly in the maintenance of pregnancy.
Figures
References
-
- Joost HG, Bell GI, Best JD, Birnbaum MJ, Charron MJ, Chen YT, Doege H, James DE, Lodish HF, Moley KH, Moley JF, Mueckler M, et al. Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am J Physiol Endocrinol Metab 2002; 282: E974 E976 - PubMed
-
- Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review). Mol Membr Biol 2001; 18: 247 256 - PubMed
-
- Wood IS, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr 2003; 89: 3 9 - PubMed
-
- Riley JK, Moley KH. Glucose utilization and the PI3-K pathway: mechanisms for cell survival in preimplantation embryos. Reproduction 2006; 131: 823 835 - PubMed
-
- Mueckler M. Family of glucose-transporter genes: implications for glucose homeostasis and diabetes. Diabetes 1990; 39: 6 11 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

