Recognition of blood group ABH type 1 determinants by the FedF adhesin of F18-fimbriated Escherichia coli
- PMID: 19208633
- PMCID: PMC2665092
- DOI: 10.1074/jbc.M807866200
Recognition of blood group ABH type 1 determinants by the FedF adhesin of F18-fimbriated Escherichia coli
Abstract
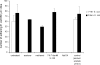
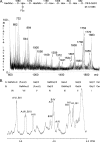
F18-fimbriated Escherichia coli are associated with porcine postweaning diarrhea and edema disease. Adhesion of F18-fimbriated bacteria to the small intestine of susceptible pigs is mediated by the minor fimbrial subunit FedF. However, the target cell receptor for FedF has remained unidentified. Here we report that F18-fimbriated E. coli selectively interact with glycosphingolipids having blood group ABH determinants on type 1 core, and blood group A type 4 heptaglycosylceramide. The minimal binding epitope was identified as the blood group H type 1 determinant (Fucalpha2Galbeta3GlcNAc), while an optimal binding epitope was created by addition of the terminal alpha3-linked galactose or N-acetylgalactosamine of the blood group B type 1 determinant (Galalpha3(Fucalpha2)Galbeta3GlcNAc) and the blood group A type 1 determinant (GalNAcalpha3(Fucalpha2)-Galbeta3GlcNAc). To assess the role of glycosphingolipid recognition by F18-fimbriated E. coli in target tissue adherence, F18-binding glycosphingolipids were isolated from the small intestinal epithelium of blood group O and A pigs and characterized by mass spectrometry and proton NMR. The only glycosphingolipid with F18-binding activity of the blood group O pig was an H type 1 pentaglycosylceramide (Fucalpha2Galbeta3GlcNAc-beta3Galbeta4Glcbeta1Cer). In contrast, the blood group A pig had a number of F18-binding glycosphingolipids, characterized as A type 1 hexaglycosylceramide (GalNAcalpha3(Fucalpha2)Galbeta3GlcNAcbeta3Galbeta4Glcbeta1Cer), A type 4 heptaglycosylceramide (GalNAcalpha3(Fucalpha2)Galbeta3GalNAcbeta3Galalpha4Galbeta4Glcbeta1Cer), A type 1 octaglycosylceramide (GalNAcalpha3(Fucalpha2)Galbeta3GlcNAcbeta3Galbeta3GlcNAcbeta3Galbeta4Glcbeta1Cer), and repetitive A type 1 nonaglycosylceramide (GalNAcalpha3(Fucalpha2)Galbeta3GalNAcalpha3-(Fucalpha2)Galbeta3GlcNAcbeta3Galbeta4Glcbeta1Cer). No blood group antigen-carrying glycosphingolipids were recognized by a mutant E. coli strain with deletion of the FedF adhesin, demonstrating that FedF is the structural element mediating binding of F18-fimbriated bacteria to blood group ABH determinants.
Figures









References
-
- Kaper, J. B., Nataro, J. P., and Mobley, H. L. (2004) Nat. Rev. Microbiol. 2 123–140 - PubMed
-
- Fairbrother, J. M., Nadeau, E., and Gyles, C. L. (2005) Anim. Health Res. Rev. 6 17–39 - PubMed
-
- Bertschinger, H., and Gyles, C. L. (1994) in Escherichia coli in Domestic Animals and Humans (Gyles, C. L. ed) pp. 193–219, CAB, Wallingford, Oxon, UK
-
- Imberechts, H., Wild, P., Charlier, G., De Greve, H., Lintermans, P., and Pohl, P. (1996) Microb. Pathog. 21 183–192 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

