JWA regulates XRCC1 and functions as a novel base excision repair protein in oxidative-stress-induced DNA single-strand breaks
- PMID: 19208635
- PMCID: PMC2665235
- DOI: 10.1093/nar/gkp054
JWA regulates XRCC1 and functions as a novel base excision repair protein in oxidative-stress-induced DNA single-strand breaks
Abstract
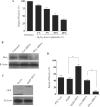
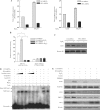
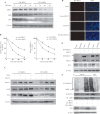
JWA was recently demonstrated to be involved in cellular responses to environmental stress including oxidative stress. Although it was found that JWA protected cells from reactive oxygen species-induced DNA damage, upregulated base excision repair (BER) protein XRCC1 and downregulated PARP-1, the molecular mechanism of JWA in regulating the repair of DNA single-strand breaks (SSBs) is still unclear. Our present studies demonstrated that a reduction in JWA protein levels in cells resulted in a decrease of SSB repair capacity and hypersensitivity to DNA-damaging agents such as methyl methanesulfonate and hydrogen peroxide. JWA functioned as a repair protein by multi-interaction with XRCC1. On the one hand, JWA was translocated into the nucleus by the carrier protein XRCC1 and co-localized with XRCC1 foci after oxidative DNA damage. On the other hand, JWA via MAPK signaling pathway regulated nuclear factor E2F1, which further transcriptionally regulated XRCC1. In addition, JWA protected XRCC1 protein from ubiquitination and degradation by proteasome. These findings indicate that JWA may serve as a novel regulator of XRCC1 in the BER protein complex to facilitate the repair of DNA SSBs.
Figures




References
-
- Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. - PubMed
-
- Caldecott KW. Mammalian DNA single-strand break repair: an X-ra(y)ted affair. Bioessays. 2001;23:447–455. - PubMed
-
- Xu YJ, Kim EY, Demple B. Excision of C-4'-oxidized deoxyribose lesions from double-stranded DNA by human apurinic/apyrimidinic endonuclease (Ape1 protein) and DNA polymerase beta. J. Biol. Chem. 1998;273:28837–28844. - PubMed
-
- Carrano AV, Minkler JL, Dillehay LE, Thompson LH. Incorporated bromodeoxyuridine enhances the sister-chromatid exchange and chromosomal aberration frequencies in an EMS-sensitive Chinese hamster cell line. Mutat. Res. 1986;162:233–239. - PubMed
-
- Dominguez I, Daza P, Natarajan AT, Cortes F. A high yield of translocations parallels the high yield of sister chromatid exchanges in the CHO mutant EM9. Mutat. Res. 1998;398:67–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

