Epigenetics meets estrogen receptor: regulation of estrogen receptor by direct lysine methylation
- PMID: 19208734
- PMCID: PMC3901989
- DOI: 10.1677/ERC-08-0305
Epigenetics meets estrogen receptor: regulation of estrogen receptor by direct lysine methylation
Abstract
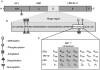
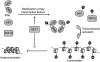
The nuclear hormone receptor estrogen receptor α (ERα) promotes cellular growth through ligand-dependent activation of specific target genes, a process which is targeted in the treatment of ERα-expressing breast cancers. ERα activity is regulated at the protein level by post-translational modifications including phosphorylation and acetylation. A study now shows that ERα can also be directly methylated at lysine 302 (K302) by SET7, a histone methyltransferase that is known to monomethylate H3K4 and is associated with transcriptional activation. It was shown that K302 methylation stabilizes ERα protein and is suggested to increase sensitivity of ERα to estrogens, enhancing transcription of estrogen response elements. Furthermore, SET7 methylation of K302 is enhanced by a breast cancer-associated mutation at K303 (K303R) in vitro. These findings provide an additional mechanism of SET7 mediated transcriptional activation, as well as potential insight into the complex regulation of ERα stability and ligand sensitivity.
Conflict of interest statement
Figures
References
-
- Chuikov S, Kurash JK, Wilson JR, Xiao B, Justin N, Ivanov GS, McKinney K, Tempst P, Prives C, Gamblin SJ, Barlev NA, Reinberg D. Regulation of p53 activity through lysine methylation. Nature. 2004;432:353–360. - PubMed
-
- Conway K, Parrish E, Edmiston SN, Tolbert D, Tse CK, Geradts J, Livasy CA, Singh H, Newman B, Millikan RC. The estrogen receptor-alpha A908G (K303R) mutation occurs at a low frequency in invasive breast tumors: results from a population-based study. Breast Cancer Research. 2005;7:871–880. - PMC - PubMed
-
- Couture JF, Collazo E, Hauk G, Trievel RC. Structural basis for the methylation site specificity of SET7/9. Nature Structural and Molecular Biology. 2006;13:140–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

