A common trafficking route for GLUT4 in cardiomyocytes in response to insulin, contraction and energy-status signalling
- PMID: 19208760
- PMCID: PMC2720923
- DOI: 10.1242/jcs.041178
A common trafficking route for GLUT4 in cardiomyocytes in response to insulin, contraction and energy-status signalling
Erratum in
- J Cell Sci. 2009 Apr 1;122(Pt 7):1054
Abstract
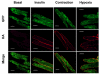
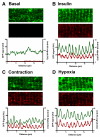
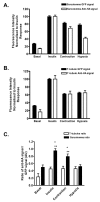
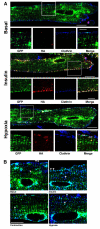
A new mouse model has been developed to study the localisation and trafficking of the glucose transporter GLUT4 in muscle. The mouse line has specific expression of a GFP and HA-epitope-tagged version of GLUT4 under the control of a muscle-specific promoter. The exofacial HA-tag has enabled fluorescent labelling of only the GLUT4 exposed at the external surface. A distinction between sarcolemma labelling and transverse-tubule labelling has also been possible because the former compartment is much more accessible to intact anti-HA antibody. By contrast, the Fab fragment of the anti-HA antibody could readily detect GLUT4 at the surface of both the sarcolemma and transverse tubules. Here, we have used this mouse model to examine the route taken by cardiomyocyte GLUT4 as it moves to the limiting external membrane surface of sarcolemma and transverse-tubules in response to insulin, contraction or activators of energy-status signalling, including hypoxia. HA-GLUT4-GFP is largely excluded from the sarcolemma and transverse-tubule membrane of cardiomyocytes under basal conditions, but is similarly trafficked to these membrane surfaces after stimulation with insulin, contraction or hypoxia. Internalisation of sarcolemma GLUT4 has been investigated by pulse-labelling surface GLUT4 with intact anti-HA antibody. At early stages of internalisation, HA-tagged GLUT4 colocalises with clathrin at puncta at the sarcolemma, indicating that in cells returning to a basal state, GLUT4 is removed from external membranes by a clathrin-mediated route. We also observed colocalisation of GLUT4 with clathrin under basal conditions. At later stages of internalisation and at steady state, anti-HA antibody labeled-GLUT4 originating from the sarcolemma was predominantly detected in a peri-nuclear compartment, indistinguishable among the specific initial stimuli. These results taken together imply a common pathway for internalisation of GLUT4, independent of the initial stimulus.
Figures
Similar articles
-
Translocation of the Na+/H+ exchanger 1 (NHE1) in cardiomyocyte responses to insulin and energy-status signalling.Biochem J. 2010 Dec 15;432(3):515-23. doi: 10.1042/BJ20100717. Biochem J. 2010. PMID: 20868366 Free PMC article.
-
Insulin stimulates fusion, but not tethering, of GLUT4 vesicles in skeletal muscle of HA-GLUT4-GFP transgenic mice.Am J Physiol Endocrinol Metab. 2012 Apr 15;302(8):E950-60. doi: 10.1152/ajpendo.00466.2011. Epub 2012 Jan 31. Am J Physiol Endocrinol Metab. 2012. PMID: 22297303 Free PMC article.
-
Insulin unmasks a COOH-terminal Glut4 epitope and increases glucose transport across T-tubules in skeletal muscle.J Cell Biol. 1996 Oct;135(2):415-30. doi: 10.1083/jcb.135.2.415. J Cell Biol. 1996. PMID: 8896598 Free PMC article.
-
In vivo imaging of GLUT4 translocation.Appl Physiol Nutr Metab. 2009 Jun;34(3):420-3. doi: 10.1139/H09-043. Appl Physiol Nutr Metab. 2009. PMID: 19448708 Review.
-
Insulin- and contraction-induced glucose transporter 4 traffic in muscle: insights from a novel imaging approach.Exerc Sport Sci Rev. 2013 Apr;41(2):77-86. doi: 10.1097/JES.0b013e318275574c. Exerc Sport Sci Rev. 2013. PMID: 23072821 Free PMC article. Review.
Cited by
-
Kinetic evidence for unique regulation of GLUT4 trafficking by insulin and AMP-activated protein kinase activators in L6 myotubes.J Biol Chem. 2010 Jan 15;285(3):1653-60. doi: 10.1074/jbc.M109.051185. Epub 2009 Nov 13. J Biol Chem. 2010. PMID: 19915010 Free PMC article.
-
Insulin controls the spatial distribution of GLUT4 on the cell surface through regulation of its postfusion dispersal.Cell Metab. 2010 Sep 8;12(3):250-9. doi: 10.1016/j.cmet.2010.08.005. Cell Metab. 2010. PMID: 20816091 Free PMC article.
-
Glucose homeostasis during short-term and prolonged exposure to high altitudes.Endocr Rev. 2015 Apr;36(2):149-73. doi: 10.1210/er.2014-1063. Epub 2015 Feb 12. Endocr Rev. 2015. PMID: 25675133 Free PMC article. Review.
-
Translocation of the Na+/H+ exchanger 1 (NHE1) in cardiomyocyte responses to insulin and energy-status signalling.Biochem J. 2010 Dec 15;432(3):515-23. doi: 10.1042/BJ20100717. Biochem J. 2010. PMID: 20868366 Free PMC article.
-
Biogenesis and regulation of insulin-responsive vesicles containing GLUT4.Curr Opin Cell Biol. 2010 Aug;22(4):506-12. doi: 10.1016/j.ceb.2010.03.012. Epub 2010 Apr 21. Curr Opin Cell Biol. 2010. PMID: 20417083 Free PMC article. Review.
References
-
- Antonescu, C. N., Diaz, M., Femia, G., Planas, J. V. and Klip, A. (2008). Clathrin and non-clathrin mediated Glut4 endocytosis in myocytes: effect of mitochondrial uncoupling. Traffic 9, 1173-1190. - PubMed
-
- Bertrand, L., Ginion, A., Beauloye, C., Hebert, A. D., Guigas, B., Hue, L. and Vanoverschelde, J. L. (2006). AMPK activation restores the stimulation of glucose uptake in an in vitro model of insulin-resistant cardiomyocytes via the activation of protein kinase B. Am. J Physiol. Heart Circ. Physiol. 291, H239-H250. - PubMed
-
- Bruning, J. C., Michael, M. D., Winnay, J. N., Hayashi, T., Horsch, D., Accili, D., Goodyear, L. J. and Kahn, C. R. (1998). A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol. Cell 2, 559-569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

