Apical membrane maturation and cellular rosette formation during morphogenesis of the zebrafish lateral line
- PMID: 19208766
- PMCID: PMC3188803
- DOI: 10.1242/jcs.032102
Apical membrane maturation and cellular rosette formation during morphogenesis of the zebrafish lateral line
Abstract
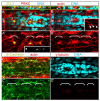
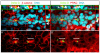
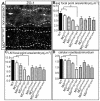
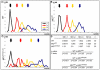
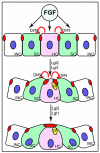
Tissue morphogenesis and cell sorting are major forces during organ development. Here, we characterize the process of tissue morphogenesis within the zebrafish lateral line primordium, a migratory sheet of cells that gives rise to the neuromasts of the posterior lateral line organ. We find that cells within this epithelial tissue constrict actin-rich membranes and enrich apical junction proteins at apical focal points. The coordinated apical membrane constriction in single Delta D-positive hair cell progenitors and in their neighbouring prospective support cells generates cellular rosettes. Live imaging reveals that cellular rosettes subsequently separate from each other and give rise to individual neuromasts. Genetic analysis uncovers an involvement of Lethal giant larvae proteins in the maturation of apical junction belts during cellular rosette formation. Our findings suggest that apical constriction of cell membranes spatially confines regions of strong cell-cell adhesion and restricts the number of tightly interconnected cells into cellular rosettes, which ensures the correct deposition of neuromasts during morphogenesis of the posterior lateral line organ.
Figures



Similar articles
-
Cell-cell signaling interactions coordinate multiple cell behaviors that drive morphogenesis of the lateral line.Cell Adh Migr. 2011 Nov-Dec;5(6):499-508. doi: 10.4161/cam.5.6.19113. Cell Adh Migr. 2011. PMID: 22274715 Free PMC article. Review.
-
Fgfr-Ras-MAPK signaling is required for apical constriction via apical positioning of Rho-associated kinase during mechanosensory organ formation.Development. 2012 Sep;139(17):3130-5. doi: 10.1242/dev.082271. Epub 2012 Jul 25. Development. 2012. PMID: 22833124 Free PMC article.
-
Atoh1a expression must be restricted by Notch signaling for effective morphogenesis of the posterior lateral line primordium in zebrafish.Development. 2010 Oct;137(20):3477-87. doi: 10.1242/dev.052761. Development. 2010. PMID: 20876657 Free PMC article.
-
Lef1 is required for progenitor cell identity in the zebrafish lateral line primordium.Development. 2011 Sep;138(18):3921-30. doi: 10.1242/dev.062554. Development. 2011. PMID: 21862556 Free PMC article.
-
Signaling pathways regulating zebrafish lateral line development.Curr Biol. 2009 May 12;19(9):R381-6. doi: 10.1016/j.cub.2009.03.057. Curr Biol. 2009. PMID: 19439264 Review.
Cited by
-
Cell-cell signaling interactions coordinate multiple cell behaviors that drive morphogenesis of the lateral line.Cell Adh Migr. 2011 Nov-Dec;5(6):499-508. doi: 10.4161/cam.5.6.19113. Cell Adh Migr. 2011. PMID: 22274715 Free PMC article. Review.
-
Lethal giant larvae 2 regulates development of the ciliated organ Kupffer's vesicle.Development. 2013 Apr;140(7):1550-9. doi: 10.1242/dev.087130. Development. 2013. PMID: 23482490 Free PMC article.
-
Zebrafish prion protein PrP2 controls collective migration process during lateral line sensory system development.PLoS One. 2014 Dec 1;9(12):e113331. doi: 10.1371/journal.pone.0113331. eCollection 2014. PLoS One. 2014. PMID: 25436888 Free PMC article.
-
Cell migration.Compr Physiol. 2012 Oct;2(4):2369-92. doi: 10.1002/cphy.c110012. Compr Physiol. 2012. PMID: 23720251 Free PMC article. Review.
-
Building the posterior lateral line system in zebrafish.Dev Neurobiol. 2012 Mar;72(3):234-55. doi: 10.1002/dneu.20962. Dev Neurobiol. 2012. PMID: 21818862 Free PMC article. Review.
References
-
- Betschinger, J., Mechtler, K. and Knoblich, J. A. (2003). The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature 422, 326-330. - PubMed
-
- Betschinger, J., Eisenhaber, F. and Knoblich, J. A. (2005). Phosphorylation-induced autoinhibition regulates the cytoskeletal protein Lethal (2) giant larvae. Curr. Biol. 15, 276-282. - PubMed
-
- Blankenship, J. T., Backovic, S. T., Sanny, J. S., Weitz, O. and Zallen, J. A. (2006). Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev. Cell 11, 459-470. - PubMed
-
- Brown, K. E., Baonza, A. and Freeman, M. (2006). Epithelial cell adhesion in the developing Drosophila retina is regulated by Atonal and the EGF receptor pathway. Dev. Biol. 300, 710-721. - PubMed
-
- Cooper, M. S., D'Amico, L. A. and Henry, C. A. (1999). Confocal microscopic analysis of morphogenetic movements. Methods Cell Biol. 59, 179-204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

