Structure and function of the human Gly1619Arg polymorphism of M6P/IGF2R domain 11 implicated in IGF2 dependent growth
- PMID: 19208780
- PMCID: PMC2659294
- DOI: 10.1677/JME-08-0154
Structure and function of the human Gly1619Arg polymorphism of M6P/IGF2R domain 11 implicated in IGF2 dependent growth
Abstract
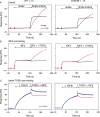
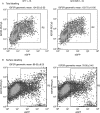
The mannose 6-phosphate/IGF 2 receptor (IGF2R) is comprised of 15 extra-cellular domains that bind IGF2 and mannose 6-phosphate ligands. IGF2R transports ligands from the Golgi to the pre-lysosomal compartment and thereafter to and from the cell surface. IGF2R regulates growth, placental development, tumour suppression and signalling. The ligand IGF2 is implicated in the growth phenotype, where IGF2R normally limits bioavailability, such that loss and gain of IGF2R results in increased and reduced growth respectively. The IGF2R exon 34 (5002A>G) polymorphism (rs629849) of the IGF2 specific binding domain has been correlated with impaired childhood growth (A/A homozygotes). We evaluated the function of the Gly1619Arg non-synonymous amino acid modification of domain 11. NMR and X-ray crystallography structures located 1619 remote from the ligand binding region of domain 11. Arg1619 was located close to the fibronectin type II (FnII) domain of domain 13, previously implicated as a modifier of IGF2 ligand binding through indirect interaction with the AB loop of the binding cleft. However, comparison of binding kinetics of IGF2R, Gly1619 and Arg1619 to either IGF2 or mannose 6-phosphate revealed no differences in 'on' and 'off' rates. Quantitative PCR, (35)S pulse chase and flow cytometry failed to demonstrate altered gene expression, protein half-life and cell membrane distribution, suggesting the polymorphism had no direct effect on receptor function. Intronic polymorphisms were identified which may be in linkage disequilibrium with rs629849 in certain populations. Other potential IGF2R polymorphisms may account for the correlation with childhood growth, warranting further functional evaluation.
Figures



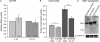


References
-
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. - PubMed
-
- Byrd JC, Devi GR, de Souza AT, Jirtle RL, MacDonald RG. Disruption of ligand binding to the insulin-like growth factor II/mannose 6-phosphate receptor by cancer-associated missense mutations. Journal of Biological Chemistry. 1999;274:24408–24416. - PubMed
-
- Byrd JC, Park JH, Schaffer BS, Garmroudi F, MacDonald RG. Dimerization of the insulin-like growth factor II/mannose 6-phosphate receptor. Journal of Biological Chemistry. 2000;275:18647–18656. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

