A systems biology understanding of the synergistic effects of arsenic sulfide and Imatinib in BCR/ABL-associated leukemia
- PMID: 19208803
- PMCID: PMC2651272
- DOI: 10.1073/pnas.0813142106
A systems biology understanding of the synergistic effects of arsenic sulfide and Imatinib in BCR/ABL-associated leukemia
Abstract
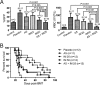
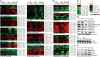
In this study, we show that combined use of Imatinib (IM) and arsenic sulfide [As(4)S(4) (AS)] exerts more profound therapeutic effects in a BCR/ABL-positive mouse model of chronic myeloid leukemia (CML) than either drug as a single agent. A systematic analysis of dynamic changes of the proteome, phosphoproteome, and transcriptome in K562 cells after AS and/or IM treatment was performed to address the mechanisms underlying this synergy. Our data indicate that AS promotes the activities of the unfolded protein reaction (UPR) and ubiquitination pathway, which could form the biochemical basis for the pharmacological effects of this compound. In this CML model, AS targets BCR/ABL through the ubiquitination of key lysine residues, leading to its proteasomal degradation, whereas IM inhibits the PI3K/AKT/mTOR pathway. Combination of the 2 agents synergistically arrests the cell cycle, decreases activity of BCR/ABL, and leads to activation of intrinsic and extrinsic apoptosis pathways through complex modifications to both transcription and protein levels. Thus, these results suggest potential clinical benefits of IM/AS combination therapy for human CML.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hehlmann R, Hochhaus A, Baccarani M. Chronic myeloid leukaemia. Lancet. 2007;370:342–350. - PubMed
-
- Shah NP, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. - PubMed
-
- Schiffer CA. BCR-ABL tyrosine kinase inhibitors for chronic myelogenous leukemia. N Engl J Med. 2007;357:258–265. - PubMed
-
- Wang ZY, Chen Z. Acute promyelocytic leukemia: From highly fatal to highly curable. Blood. 2008;111:2505–2515. - PubMed
-
- Du YZ, et al. Coordination of intrinsic, extrinsic, and endoplasmic reticulum-mediated apoptosis by imatinib mesylate combined with arsenic trioxide in chronic myeloid leukemia. Blood. 2006;107:1582–1590. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

