Haploinsufficiency for Pten and Serotonin transporter cooperatively influences brain size and social behavior
- PMID: 19208814
- PMCID: PMC2644151
- DOI: 10.1073/pnas.0804428106
Haploinsufficiency for Pten and Serotonin transporter cooperatively influences brain size and social behavior
Abstract
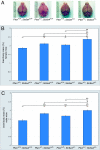
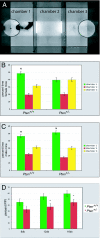
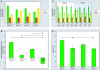
Autism spectrum disorder (ASD) is a heterogeneous group of neurodevelopmental disorders that share deficits in sociability, communication, and restrictive and repetitive interests. ASD is likely polygenic in origin in most cases, but we presently lack an understanding of the relationships between ASD susceptibility genes and the neurobiological and behavioral phenotypes of ASD. Two genes that have been implicated as conferring susceptibility to ASD are PTEN and Serotonin transporter (SLC6A4). The PI3K and serotonin pathways, in which these genes respectively act, are both potential biomarkers for ASD diagnosis and treatment. Biochemical evidence exists for an interaction between these pathways; however, the relevance of this for the pathogenesis of ASD is unclear. We find that Pten haploinsufficient (Pten(+/-)) mice are macrocephalic, and this phenotype is exacerbated in Pten(+/-); Slc6a4(+/-) mice. Furthermore, female Pten(+/-) mice are impaired in social approach behavior, a phenotype that is exacerbated in female Pten(+/-); Slc6a4(+/-) mice. While increased brain size correlates with decreased sociability across these genotypes in females, within each genotype increased brain size correlates with increased sociability, suggesting that epigenetic influences interact with genetic factors in influencing the phenotype. These findings provide insight into an interaction between two ASD candidate genes during brain development and point toward the use of compound mutant mice to validate biomarkers for ASD against biological and behavioral phenotypes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Decreased aggression and increased repetitive behavior in Pten haploinsufficient mice.Genes Brain Behav. 2015 Feb;14(2):145-57. doi: 10.1111/gbb.12192. Epub 2015 Feb 10. Genes Brain Behav. 2015. PMID: 25561290
-
SLC6A4 markers modulate platelet 5-HT level and specific behaviors of autism: a study from an Indian population.Prog Neuropsychopharmacol Biol Psychiatry. 2015 Jan 2;56:196-206. doi: 10.1016/j.pnpbp.2014.09.004. Epub 2014 Sep 28. Prog Neuropsychopharmacol Biol Psychiatry. 2015. PMID: 25261775
-
Pten haploinsufficient mice show broad brain overgrowth but selective impairments in autism-relevant behavioral tests.Hum Mol Genet. 2014 Jul 1;23(13):3490-505. doi: 10.1093/hmg/ddu057. Epub 2014 Feb 4. Hum Mol Genet. 2014. PMID: 24497577
-
The serotonin system in autism spectrum disorder: From biomarker to animal models.Neuroscience. 2016 May 3;321:24-41. doi: 10.1016/j.neuroscience.2015.11.010. Epub 2015 Nov 11. Neuroscience. 2016. PMID: 26577932 Free PMC article. Review.
-
Behavioral neuroscience of autism.Neurosci Biobehav Rev. 2020 Mar;110:60-76. doi: 10.1016/j.neubiorev.2019.04.012. Epub 2019 May 3. Neurosci Biobehav Rev. 2020. PMID: 31059731 Review.
Cited by
-
A randomized double-blind controlled trial of everolimus in individuals with PTEN mutations: Study design and statistical considerations.Contemp Clin Trials Commun. 2021 Feb 6;21:100733. doi: 10.1016/j.conctc.2021.100733. eCollection 2021 Mar. Contemp Clin Trials Commun. 2021. PMID: 33644493 Free PMC article.
-
Germline nuclear-predominant Pten murine model exhibits impaired social and perseverative behavior, microglial activation, and increased oxytocinergic activity.Mol Autism. 2021 Jun 4;12(1):41. doi: 10.1186/s13229-021-00448-4. Mol Autism. 2021. PMID: 34088332 Free PMC article.
-
Pten Mutations Alter Brain Growth Trajectory and Allocation of Cell Types through Elevated β-Catenin Signaling.J Neurosci. 2015 Jul 15;35(28):10252-67. doi: 10.1523/JNEUROSCI.5272-14.2015. J Neurosci. 2015. PMID: 26180201 Free PMC article.
-
A standardized social preference protocol for measuring social deficits in mouse models of autism.Nat Protoc. 2020 Oct;15(10):3464-3477. doi: 10.1038/s41596-020-0382-9. Epub 2020 Sep 7. Nat Protoc. 2020. PMID: 32895524 Free PMC article.
-
Serotonin-related pathways and developmental plasticity: relevance for psychiatric disorders.Dialogues Clin Neurosci. 2014 Mar;16(1):29-41. doi: 10.31887/DCNS.2014.16.1/adayer. Dialogues Clin Neurosci. 2014. PMID: 24733969 Free PMC article. Review.
References
-
- Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–192. - PubMed
-
- Goffin A, Hoefsloot LH, Bosgoed E, Swillen A, Fryns JP. PTEN mutation in a family with Cowden syndrome and autism. Am J Med Genet. 2001;105:521–524. - PubMed
-
- Herman GE, et al. Increasing knowledge of PTEN germline mutations: two additional patients with autism and macrocephaly. Am J Med Genet A. 2007;143:589–593. - PubMed
-
- Herman GE, et al. Genetic testing in autism: How much is enough? Genet Med. 2007;9:268–274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

