Tumor and vascular targeting of a novel oncolytic measles virus retargeted against the urokinase receptor
- PMID: 19208845
- PMCID: PMC2739455
- DOI: 10.1158/0008-5472.CAN-08-2628
Tumor and vascular targeting of a novel oncolytic measles virus retargeted against the urokinase receptor
Abstract
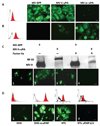
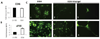
Oncolytic measles virus (MV) induces cell fusion and cytotoxicity in a CD46-dependent manner. Development of fully retargeted oncolytic MVs would improve tumor selectivity. The urokinase-type plasminogen activator receptor (uPAR) is a tumor and stromal target overexpressed in multiple malignancies. MV-H glycoproteins fully retargeted to either human or murine uPAR were engineered and their fusogenic activity was determined. Recombinant human (MV-h-uPA) and murine (MV-m-uPA) uPAR-retargeted MVs expressing enhanced green fluorescent protein (eGFP) were rescued and characterized. Viral expression of chimeric MV-H was shown by reverse transcription-PCR and Western blot. In vitro viral replication was comparable to MV-GFP control. The receptor and species specificity of MV-uPAs was shown in human and murine cells with different levels of uPAR expression. Removal of the NH(2)-terminal fragment ligand from MV-uPA by factor X(a) treatment ablated the MV-uPA functional activity. Cytotoxicity was shown in uPAR-expressing human and murine cells. MV-h-uPA efficiently infected human endothelial cells and capillary tubes in vitro. I.v. administration of MV-h-uPA delayed tumor growth and prolonged survival in the MDA-MB-231 breast cancer xenograft model. Viral tumor targeting was confirmed by immunohistochemistry. MV-m-uPA transduced murine mammary tumors (4T1) in vivo after intratumor administration. MV-m-uPA targeted murine tumor vasculature after systemic administration, as shown by dual (CD31 and MV-N) staining of tumor capillaries in the MDA-MB-231 model. In conclusion, MV-uPA is a novel oncolytic MV associated with potent and specific antitumor effects and tumor vascular targeting. This is the first retargeted oncolytic MV able to replicate in murine cells and target tumor vasculature in a uPAR-dependent manner.
Figures




Similar articles
-
Molecular Effects of Stromal-Selective Targeting by uPAR-Retargeted Oncolytic Virus in Breast Cancer.Mol Cancer Res. 2017 Oct;15(10):1410-1420. doi: 10.1158/1541-7786.MCR-17-0016. Epub 2017 Jul 5. Mol Cancer Res. 2017. PMID: 28679779 Free PMC article.
-
In vivo anti-metastatic effects of uPAR retargeted measles virus in syngeneic and xenograft models of mammary cancer.Breast Cancer Res Treat. 2015 Jan;149(1):99-108. doi: 10.1007/s10549-014-3236-8. Epub 2014 Dec 18. Breast Cancer Res Treat. 2015. PMID: 25519042 Free PMC article.
-
In vivo safety, biodistribution and antitumor effects of uPAR retargeted oncolytic measles virus in syngeneic cancer models.Gene Ther. 2014 Mar;21(3):289-97. doi: 10.1038/gt.2013.84. Epub 2014 Jan 16. Gene Ther. 2014. PMID: 24430235 Free PMC article.
-
Therapeutic potential of oncolytic measles virus: promises and challenges.Clin Pharmacol Ther. 2010 Nov;88(5):620-5. doi: 10.1038/clpt.2010.211. Epub 2010 Sep 29. Clin Pharmacol Ther. 2010. PMID: 20881957 Review.
-
Potential and clinical translation of oncolytic measles viruses.Expert Opin Biol Ther. 2017 Mar;17(3):353-363. doi: 10.1080/14712598.2017.1288713. Expert Opin Biol Ther. 2017. PMID: 28129716 Free PMC article. Review.
Cited by
-
Bi- and Tri-Specific T Cell Engager-Armed Oncolytic Viruses: Next-Generation Cancer Immunotherapy.Biomedicines. 2020 Jul 10;8(7):204. doi: 10.3390/biomedicines8070204. Biomedicines. 2020. PMID: 32664210 Free PMC article. Review.
-
Natural oncolytic activity of live-attenuated measles virus against human lung and colorectal adenocarcinomas.Biomed Res Int. 2013;2013:387362. doi: 10.1155/2013/387362. Epub 2013 Mar 17. Biomed Res Int. 2013. PMID: 23586034 Free PMC article.
-
A new strategy for treating colorectal cancer: Regulating the influence of intestinal flora and oncolytic virus on interferon.Mol Ther Oncolytics. 2023 Aug 24;30:254-274. doi: 10.1016/j.omto.2023.08.010. eCollection 2023 Sep 21. Mol Ther Oncolytics. 2023. PMID: 37701850 Free PMC article. Review.
-
Designing and building oncolytic viruses.Future Virol. 2017 Apr;12(4):193-213. doi: 10.2217/fvl-2016-0129. Epub 2017 Mar 31. Future Virol. 2017. PMID: 29387140 Free PMC article. Review.
-
Attenuated measles virus controls pediatric acute B-lineage lymphoblastic leukemia in NOD/SCID mice.Haematologica. 2014 Jun;99(6):1050-61. doi: 10.3324/haematol.2013.087205. Epub 2014 Apr 3. Haematologica. 2014. PMID: 24700491 Free PMC article.
References
-
- Fielding AK. Measles as a potential oncolytic virus. Rev Med Virol. 2005;15:135–142. - PubMed
-
- Nakamura T, Russell SJ. Oncolytic measles viruses for cancer therapy. Expert Opin Biol Ther. 2004;4:1685–1692. - PubMed
-
- Russell SJ. RNA viruses as virotherapy agents. Cancer Gene Ther. 2002;9:961–966. - PubMed
-
- Bateman A, Bullough F, Murphy S, et al. Fusogenic membrane glycoproteins as a novel class of genes for the local and immune-mediated control of tumor growth. Cancer Res. 2000;60:1492–1497. - PubMed
-
- Vile RG, Russell SJ, Lemoine NR. Cancer gene therapy: hard lessons and new courses. Gene Ther. 2000;7:2–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

