Phytochrome A mediates rapid red light-induced phosphorylation of Arabidopsis FAR-RED ELONGATED HYPOCOTYL1 in a low fluence response
- PMID: 19208901
- PMCID: PMC2660616
- DOI: 10.1105/tpc.108.061259
Phytochrome A mediates rapid red light-induced phosphorylation of Arabidopsis FAR-RED ELONGATED HYPOCOTYL1 in a low fluence response
Abstract
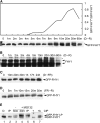
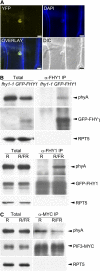
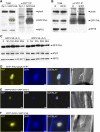
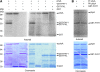
Phytochrome A (phyA) is the primary photoreceptor for mediating the far-red high irradiance response in Arabidopsis thaliana. FAR-RED ELONGATED HYPOCOTYL1 (FHY1) and its homolog FHY1-LIKE (FHL) define two positive regulators in the phyA signaling pathway. These two proteins have been reported to be essential for light-regulated phyA nuclear accumulation through direct physical interaction with phyA. Here, we report that FHY1 protein is phosphorylated rapidly after exposure to red light. Subsequent exposure to far-red light after the red light pulse reverses FHY1 phosphorylation. Such a phenomenon represents a classical red/far-red reversible low fluence response. The phosphorylation of FHY1 depends on functioning phyA but not on other phytochromes and cryptochromes. Furthermore, we demonstrate that FHY1 and FHL directly interact with phyA by bimolecular fluorescence complementation and that both FHY1 and FHL interact more stably with the Pr form of phyA in Arabidopsis seedlings by coimmunoprecipitation. Finally, in vitro kinase assays confirmed that a recombinant phyA is able to robustly phosphorylate FHY1. Together, our results suggest that phyA may differentially regulate FHY1 and FHL activity through direct physical interaction and red/far-red light reversible phosphorylation to fine-tune their degradation rates and resulting light responses.
Figures



References
-
- Ahmad, M., Jarillo, J.A., Smirnova, O., and Cashmore, A.R. (1998. b). The CRY1 blue light photoreceptor of Arabidopsis interacts with phytochrome A in vitro. Mol. Cell 1 939–948. - PubMed
-
- Al-Sady, B., Ni, W., Kircher, S., Schafer, E., and Quail, P.H. (2006). Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol. Cell 23 439–446. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

