Accelerated postnatal growth increases lipogenic gene expression and adipocyte size in low-birth weight mice
- PMID: 19208909
- PMCID: PMC2671035
- DOI: 10.2337/db08-1266
Accelerated postnatal growth increases lipogenic gene expression and adipocyte size in low-birth weight mice
Abstract
Objective: To characterize the hormonal milieu and adipose gene expression in response to catch-up growth (CUG), a growth pattern associated with obesity and diabetes risk, in a mouse model of low birth weight (LBW).
Research design and methods: ICR mice were food restricted by 50% from gestational days 12.5-18.5, reducing offspring birth weight by 25%. During the suckling period, dams were either fed ad libitum, permitting CUG in offspring, or food restricted, preventing CUG. Offspring were killed at age 3 weeks, and gonadal fat was removed for RNA extraction, array analysis, RT-PCR, and evaluation of cell size and number. Serum insulin, thyroxine (T4), corticosterone, and adipokines were measured.
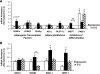
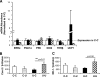
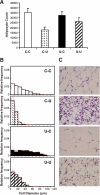
Results: At age 3 weeks, LBW mice with CUG (designated U-C) had body weight comparable with controls (designated C-C); weight was reduced by 49% in LBW mice without CUG (designated U-U). Adiposity was altered by postnatal nutrition, with gonadal fat increased by 50% in U-C and decreased by 58% in U-U mice (P < 0.05 vs. C-C mice). Adipose expression of the lipogenic genes Fasn, AccI, Lpin1, and Srebf1 was significantly increased in U-C compared with both C-C and U-U mice (P < 0.05). Mitochondrial DNA copy number was reduced by >50% in U-C versus U-U mice (P = 0.014). Although cell numbers did not differ, mean adipocyte diameter was increased in U-C and reduced in U-U mice (P < 0.01).
Conclusions: CUG results in increased adipose tissue lipogenic gene expression and adipocyte diameter but not increased cellularity, suggesting that catch-up fat is primarily associated with lipogenesis rather than adipogenesis in this murine model.
Figures
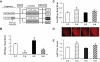
 , C-U; ■, U-C; ▨, U-U.
, C-U; ■, U-C; ▨, U-U.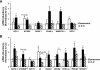
Comment in
-
Adipose tissue plasticity in catch-up-growth trajectories to metabolic syndrome: hyperplastic versus hypertrophic catch-up fat.Diabetes. 2009 May;58(5):1037-9. doi: 10.2337/db09-0290. Diabetes. 2009. PMID: 19401433 Free PMC article. No abstract available.
References
-
- Woo M, Patti ME: Diabetes risk begins in utero. Cell Metab 2008; 8: 5– 7 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

