Enhancement of oncolytic properties of recombinant newcastle disease virus through antagonism of cellular innate immune responses
- PMID: 19209145
- PMCID: PMC2835121
- DOI: 10.1038/mt.2008.286
Enhancement of oncolytic properties of recombinant newcastle disease virus through antagonism of cellular innate immune responses
Abstract
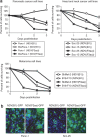
Newcastle disease virus (NDV) has been previously shown to possess oncolytic activity, causing specific lysis of cancerous but not normal cells. Here we show that despite these findings, the oncolytic efficiency of naturally occurring NDV strains can still be relatively low, as many tumors exhibit strong innate immune responses that suppress viral replication and spread. To overcome this problem, we generated a recombinant fusogenic NDV expressing influenza NS1 protein, a protein exhibiting interferon (IFN)-antagonist and antiapoptotic functions in human and mouse cells. Interestingly, the resultant virus was dramatically enhanced in its ability to form syncytia, lyse a variety of human and mouse tumor cell lines, and suppressed the induction of the cellular IFN responses. Using the aggressive syngeneic murine melanoma model, we show that the NDV-NS1 virus is more effective than virus not expressing NS1 in clearing the established footpad tumors and results in higher overall long-term animal survival. In addition, mice treated with NDV-NS1 exhibited no signs of toxicity to the virus and developed tumor-specific cytotoxic T lymphocyte (CTL) responses. These findings demonstrate that modulation of innate immune responses by NDV results in enhancement of its oncolytic properties and warrant further investigation of this strategy in design of oncolytic NDV vectors against human tumors.
Figures

Similar articles
-
Type I interferon-sensitive recombinant newcastle disease virus for oncolytic virotherapy.J Virol. 2010 Apr;84(8):3835-44. doi: 10.1128/JVI.01553-09. Epub 2010 Feb 10. J Virol. 2010. PMID: 20147405 Free PMC article.
-
Different responses of human pancreatic adenocarcinoma cell lines to oncolytic Newcastle disease virus infection.Cancer Gene Ther. 2014 Jan;21(1):24-30. doi: 10.1038/cgt.2013.78. Epub 2014 Jan 3. Cancer Gene Ther. 2014. PMID: 24384773
-
Recombinant oncolytic Newcastle disease virus displays antitumor activities in anaplastic thyroid cancer cells.BMC Cancer. 2018 Jul 18;18(1):746. doi: 10.1186/s12885-018-4522-3. BMC Cancer. 2018. PMID: 30021550 Free PMC article.
-
[Progress in using Newcastle disease virus for tumor therapy: a review].Sheng Wu Gong Cheng Xue Bao. 2010 Aug;26(8):1031-6. Sheng Wu Gong Cheng Xue Bao. 2010. PMID: 21090105 Review. Chinese.
-
Oncolytic Newcastle disease virus as a prospective anti-cancer therapy. A biologic agent with potential to break therapy resistance.Expert Opin Biol Ther. 2015;15(12):1757-71. doi: 10.1517/14712598.2015.1088000. Epub 2015 Oct 5. Expert Opin Biol Ther. 2015. PMID: 26436571 Review.
Cited by
-
CD8(+) T-cell Immune Evasion Enables Oncolytic Virus Immunotherapy.EBioMedicine. 2016 Jan 19;5:59-67. doi: 10.1016/j.ebiom.2016.01.022. eCollection 2016 Mar. EBioMedicine. 2016. PMID: 27077112 Free PMC article.
-
Oncolytic Sindbis virus targets tumors defective in the interferon response and induces significant bystander antitumor immunity in vivo.Mol Ther. 2012 Feb;20(2):298-305. doi: 10.1038/mt.2011.245. Epub 2011 Nov 8. Mol Ther. 2012. PMID: 22068428 Free PMC article.
-
Mitophagy promotes replication of oncolytic Newcastle disease virus by blocking intrinsic apoptosis in lung cancer cells.Oncotarget. 2014 Aug 15;5(15):6365-74. doi: 10.18632/oncotarget.2219. Oncotarget. 2014. PMID: 25051374 Free PMC article.
-
Recent advances in oncolytic virus design.Clin Transl Oncol. 2011 Apr;13(4):229-39. doi: 10.1007/s12094-011-0647-4. Clin Transl Oncol. 2011. PMID: 21493183 Review.
-
The tumor suppressive effect and apoptotic mechanism of TRAIL gene-containing recombinant NDV in TRAIL-resistant colorectal cancer HT-29 cells and TRAIL-nonresistant HCT116 cells, with each cell bearing a mouse model.Cancer Med. 2023 Oct;12(20):20380-20395. doi: 10.1002/cam4.6622. Epub 2023 Oct 16. Cancer Med. 2023. PMID: 37843231 Free PMC article.
References
-
- Alexander DJ. Newcastle Disease, Newcastle Disease Virus—An Avian Paramyxovirus. Kluwer Academic: Dordrecht, the Netherlands; 1988. pp. 1–22.
-
- Sinkovics JG., and , Horvath JC. Newcastle disease virus (NDV): brief history of its oncolytic strains. J Clin Virol. 2000;16:1–15. - PubMed
-
- Karcher J, Dyckhoff G, Beckhove P, Reisser C, Brysch M, Ziouta Y, et al. Antitumor vaccination in patients with head and neck squamous cell carcinomas with autologous virus-modified tumor cells. Cancer Res. 2004;64:8057–8061. - PubMed
-
- Schneider T, Gerhards R, Kirches E., and , Firsching R. Preliminary results of active specific immunization with modified tumor cell vaccine in glioblastoma multiforme. J Neurooncol. 2001;53:39–46. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

