Adoptive immunotherapy of disseminated leukemia with TCR-transduced, CD8+ T cells expressing a known endogenous TCR
- PMID: 19209146
- PMCID: PMC2730935
- DOI: 10.1038/mt.2008.300
Adoptive immunotherapy of disseminated leukemia with TCR-transduced, CD8+ T cells expressing a known endogenous TCR
Abstract
Adoptive T-cell immunotherapy has shown promise in the treatment of human malignancies, but the challenge of isolating T cells with high avidity for tumor antigens in each patient has limited application of this approach. The transfer into T cells of T-cell receptor (TCR) genes encoding high-affinity TCRs recognizing defined tumor-associated antigens can potentially circumvent this obstacle. Using a well-characterized murine model of adoptive T-cell immunotherapy for widely disseminated leukemia, we demonstrate that TCR gene-modified T cells can cure mice of disseminated tumor. One goal of such adoptive therapy is to establish a persistent memory response to prevent recurrence; however, long-term function of transferred TCR-transduced T cells is limited due to reduced expression of the introduced TCR in vivo in quiescent resting T cells. However, by introducing the TCR into a cell with a known endogenous specificity, activation of these T cells by stimulation through the endogenous TCR can be used to increase expression of the introduced TCR, potentially providing a strategy to increase the total number of tumor-reactive T cells in the host and restore more potent antitumor activity.
Figures

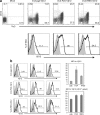
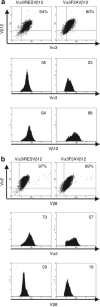


References
-
- Ho WY, Blattman JN, Dossett ML, Yee C., and , Greenberg PD. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3:431–437. - PubMed
-
- Riddell SR., and , Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128:189–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

