Epidermal homeostasis: a balancing act of stem cells in the skin
- PMID: 19209183
- PMCID: PMC2760218
- DOI: 10.1038/nrm2636
Epidermal homeostasis: a balancing act of stem cells in the skin
Abstract
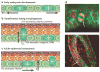
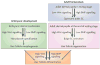
The skin epidermis and its array of appendages undergo ongoing renewal by a process called homeostasis. Stem cells in the epidermis have a crucial role in maintaining tissue homeostasis by providing new cells to replace those that are constantly lost during tissue turnover or following injury. Different resident skin stem cell pools contribute to the maintenance and repair of the various epidermal tissues of the skin, including interfollicular epidermis, hair follicles and sebaceous glands. Interestingly, the basic mechanisms and signalling pathways that orchestrate epithelial morphogenesis in the skin are reused during adult life to regulate skin homeostasis.
Figures
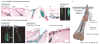
References
-
- Koster MI, Roop DR. Mechanisms regulating epithelial stratification. Annu Rev Cell Dev Biol. 2007;23:93–113. - PubMed
-
- Fuchs E, Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980;19:1033–1042. - PubMed
-
- Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nature Rev Mol Cell Biol. 2005;6:328–340. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

