Empathy is moderated by genetic background in mice
- PMID: 19209221
- PMCID: PMC2633046
- DOI: 10.1371/journal.pone.0004387
Empathy is moderated by genetic background in mice
Abstract
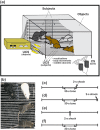
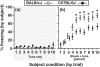
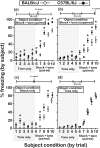
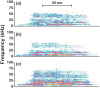
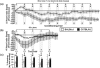
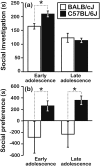
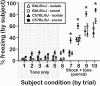
Empathy, as originally defined, refers to an emotional experience that is shared among individuals. When discomfort or alarm is detected in another, a variety of behavioral responses can follow, including greater levels of nurturing, consolation or increased vigilance towards a threat. Moreover, changes in systemic physiology often accompany the recognition of distressed states in others. Employing a mouse model of cue-conditioned fear, we asked whether exposure to conspecific distress influences how a mouse subsequently responds to environmental cues that predict this distress. We found that mice are responsive to environmental cues that predict social distress, that their heart rate changes when distress vocalizations are emitted from conspecifics, and that genetic background substantially influences the magnitude of these responses. Specifically, during a series of pre-exposure sessions, repeated experiences of object mice that were exposed to a tone-shock (CS-UCS) contingency resulted in heart rate deceleration in subjects from the gregarious C57BL/6J (B6) strain, but not in subjects from the less social BALB/cJ (BALB) strain. Following the pre-exposure sessions, subjects were individually presented with the CS-only for 5 consecutive trials followed by 5 consecutive pairings of the CS with the UCS. Pre-exposure to object distress increased the freezing responses of B6 mice, but not BALB mice, on both the CS-only and the CS-UCS trials. These physiological and behavioral responses of B6 mice to social distress parallel features of human empathy. Our paradigm thus has construct and face validity with contemporary views of empathy, and provides unequivocal evidence for a genetic contribution to the expression of empathic behavior.
Conflict of interest statement
Figures




References
-
- Mateo JM. The development of alarm-call response behaviour in free-living juvenile Belding's ground squirrels. Anim Behav. 1996;52:489–505. - PubMed
-
- Baack JK, Switzer PV. Alarm calls affect foraging behavior in eastern chipmunks. Ethol. 2000;106:1057–1066.
-
- Ehret G, Bernecker C. Low-frequency sound communication by mouse pups (Mus musculus): wriggling calls release maternal behaviour. Anim Behav. 1986;34:821–830.
-
- D'Amato FR, Scalera E, Sarli C, Moles A. Pups call, mothers rush: Does maternal responsiveness affect the amount of ultrasonic vocalizations in mouse pups. Behav Genetics. 2005;35:103–112. Also see Behav Genetics 36: pp.471 (Erratum) - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

