Life and death of an influential passenger: Wolbachia and the evolution of CI-modifiers by their hosts
- PMID: 19209229
- PMCID: PMC2635967
- DOI: 10.1371/journal.pone.0004425
Life and death of an influential passenger: Wolbachia and the evolution of CI-modifiers by their hosts
Abstract
Background: Wolbachia are intracellular bacteria widely distributed among arthropods and nematodes. In many insect species these bacteria induce a cytoplasmic incompatibility (CI) between sperm of infected males and eggs of uninfected females. From an evolutionary point of view, CI is puzzling: In order to induce this modification-rescue system, Wolbachia affect sperm of infected males even though Wolbachia are only transmitted maternally. Phylogenetic studies of Wolbachia and hosts show that the bacteria rarely cospeciate with their hosts, indicating that infections are lost in host species. However, the mechanisms leading to Wolbachia loss are not well understood.
Results: Using a population genetic model, we investigate the spread of host mutants that enhance or repress Wolbachia action by affecting either bacterial transmission or the level of CI. We show that host mutants that decrease CI-levels in males (e.g. by reducing Wolbachia-density during spermatogenesis) spread, even at cost to mutant males. Increase of these mutants can lead to loss of Wolbachia infections, either as a direct consequence of their increase or in a step-wise manner, and we derive analytically a threshold penetrance above which a mutation's spread leads to extinction of Wolbachia. Selection on host modifiers is sexually antagonistic in that, conversely, host mutants that enhance Wolbachia in females are favoured whereas suppressors are not.
Conclusions: Our results indicate that Wolbachia is likely to be lost from host populations on long evolutionary time scales due to reduction of CI levels in males. This can occur either by evolution of single host modifiers with large effects or through accumulation of several modifier alleles with small effects on Wolbachia action, even at cost to mutant males and even if infected hosts do not incur fecundity costs. This possibility is consistent with recent findings and may help to explain the apparent short evolutionary persistence times of Wolbachia in many host systems.
Conflict of interest statement
Figures
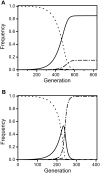
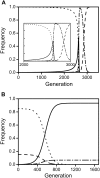
 , as larger values of −d all lead to t
eff. = 1. Other parameters were c = 0 and f = 0.
, as larger values of −d all lead to t
eff. = 1. Other parameters were c = 0 and f = 0.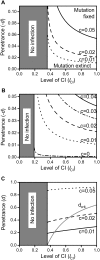
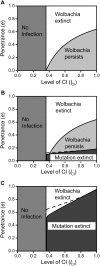
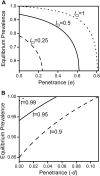
 (where t
eff. becomes 1) and between 0 and 1 in subfigure c. The grey line in 5c depicts the critical penetrance level d
crit. above which Wolbachia is driven to extinction by the mutation's spread. Other parameters were e = 0 and f = 0.
(where t
eff. becomes 1) and between 0 and 1 in subfigure c. The grey line in 5c depicts the critical penetrance level d
crit. above which Wolbachia is driven to extinction by the mutation's spread. Other parameters were e = 0 and f = 0.
Similar articles
-
A Wolbachia infection from Drosophila that causes cytoplasmic incompatibility despite low prevalence and densities in males.Heredity (Edinb). 2019 Apr;122(4):428-440. doi: 10.1038/s41437-018-0133-7. Epub 2018 Aug 23. Heredity (Edinb). 2019. PMID: 30139962 Free PMC article.
-
Wolbachia-induced cytoplasmic incompatibility is associated with decreased Hira expression in male Drosophila.PLoS One. 2011 Apr 29;6(4):e19512. doi: 10.1371/journal.pone.0019512. PLoS One. 2011. PMID: 21559343 Free PMC article.
-
A new model and method for understanding Wolbachia-induced cytoplasmic incompatibility.PLoS One. 2011 May 10;6(5):e19757. doi: 10.1371/journal.pone.0019757. PLoS One. 2011. PMID: 21572955 Free PMC article.
-
The Biochemistry of Cytoplasmic Incompatibility Caused by Endosymbiotic Bacteria.Genes (Basel). 2020 Jul 25;11(8):852. doi: 10.3390/genes11080852. Genes (Basel). 2020. PMID: 32722516 Free PMC article. Review.
-
The genetics and cell biology of Wolbachia-host interactions.Annu Rev Genet. 2008;42:683-707. doi: 10.1146/annurev.genet.41.110306.130354. Annu Rev Genet. 2008. PMID: 18713031 Review.
Cited by
-
Reproductive parasitism: maternally inherited symbionts in a biparental world.Cold Spring Harb Perspect Biol. 2015 May 1;7(5):a017699. doi: 10.1101/cshperspect.a017699. Cold Spring Harb Perspect Biol. 2015. PMID: 25934011 Free PMC article. Review.
-
Modeling the indirect effect of Wolbachia on the infection dynamics of horizontally transmitted viruses.Front Microbiol. 2015 Apr 28;6:378. doi: 10.3389/fmicb.2015.00378. eCollection 2015. Front Microbiol. 2015. PMID: 25972858 Free PMC article.
-
Suppression of cytoplasmic incompatibility in the leaf-mining fly Liriomyza sativae with a nuclear Wolbachia insert.R Soc Open Sci. 2025 May 21;12(5):242137. doi: 10.1098/rsos.242137. eCollection 2025 May. R Soc Open Sci. 2025. PMID: 40400517 Free PMC article.
-
Recent speciation in three closely related sympatric specialists: inferences using multi-locus sequence, post-mating isolation and endosymbiont data.PLoS One. 2011;6(11):e27834. doi: 10.1371/journal.pone.0027834. Epub 2011 Nov 15. PLoS One. 2011. PMID: 22110767 Free PMC article.
-
Comparative Genomics of Two Closely Related Wolbachia with Different Reproductive Effects on Hosts.Genome Biol Evol. 2016 Jun 3;8(5):1526-42. doi: 10.1093/gbe/evw096. Genome Biol Evol. 2016. PMID: 27189996 Free PMC article.
References
-
- Werren JH, Zhang W, Guo LR. Evolution and Phylogeny of Wolbachia: Reproductive Parasites of Arthropods. Proc R Soc London, Ser B. 1995;261:55–63. - PubMed
-
- West SA, Cook JM, Werren JH, Godfray HCJ. Wolbachia in two insect host-parasitoid communities. Mol Ecol. 2000;7:1457–1465. - PubMed
-
- Laven H. Eradication of Culex pipiens fatigans through Cytoplasmic Incompatibility. Nature. 1967;216:383–384. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

